-
Name
2-Fluoro-6-nitrotoluene
- EINECS 212-203-3
- CAS No. 769-10-8
- Article Data11
- CAS DataBase
- Density 1.274 g/cm3
- Solubility
- Melting Point 6.5-7 ºC
- Formula C7H6FNO2
- Boiling Point 224.3 ºC at 760 mmHg
- Molecular Weight 155.129
- Flash Point 88.9 ºC
- Transport Information UN 2810
- Appearance colorless to light yellow liquid
- Safety 26-36-28-24/25
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 1-Fluor-2-methyl-3-nitrobenzene;Benzene, 1-fluoro-2-methyl-3-nitro-;
- PSA 45.82000
- LogP 2.56550
Synthetic route
-
-
95-52-3
2-Fluorotoluene

-
A
-
455-88-9
5-nitro-2-fluorotoluene

-
B
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| With nitric acid |

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| With sand at 300℃; | |
| at 250℃; |

| Conditions | Yield |
|---|---|
| Ueberfuehrung in das Diazopiperidid und Erhitzen mit Fluorwasserstoff-Fluorkalium; | |
| (i) NaNO2, HBF4, (ii) (thermolysis); Multistep reaction; | |
| (i) (diazotization), BF4-, (ii) (pyrolysis); Multistep reaction; | |
| Stage #1: 3-nitro-o-tolylamine With sulfuric acid; sodium nitrite at 0 - 20℃; for 2h; Stage #2: With copper (I) fluoride Sandmeyer reaction; Further stages.; |

| Conditions | Yield |
|---|---|
| With nitric acid |

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride at 25℃; for 0.81h; electrochemical synthesis: Pt electrodes, applied potential 2.40 V, frequency square wave alternating current of 0.033 Hz, passed charge of 2.00 F mol-1; Yield given. Yields of byproduct given; |
-
-
95-52-3
2-Fluorotoluene

-
-
7697-37-2
nitric acid

-
A
-
455-88-9
5-nitro-2-fluorotoluene

-
B
-
1427-07-2
2-fluoro-4-nitrotoluene

-
C
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene


-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| at 150 - 250℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 4>2S 2: (i) (diazotization), BF4-, (ii) (pyrolysis) View Scheme |
-
-
19406-51-0
4-Amino-2,6-dinitrotoluene

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) (diazotization), (ii) (reduction) 2: 4>2S 3: (i) (diazotization), BF4-, (ii) (pyrolysis) View Scheme |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| With sulfuric acid; uronium nitrate at 0 - 20℃; Nitration; | 94% |
-
-
50-00-0
formaldehyd

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
118665-03-5
2-(2-fluoro-6-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; dimethyl sulfoxide for 72h; Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N,N-dimethyl-formamide at 180℃; under 6000.6 - 7500.75 Torr; for 0.333333h; microwave irradiation; | 85% |
| With triethylamine In N,N-dimethyl-formamide at 110℃; for 20h; |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; tetrabutyl ammonium fluoride; potassium carbonate In tetrahydrofuran for 0.5h; Ambient temperature; | 82% |

| Conditions | Yield |
|---|---|
| With triethylphosphine In m-xylene at 120℃; for 16h; Inert atmosphere; Schlenk technique; Sealed tube; | 82% |
-
-
553-90-2
Dimethyl oxalate

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
912819-08-0
methyl 3-(2-fluoro-6-nitrophenyl)-2-oxopropanoate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 25℃; for 19h; | 80% |
| Stage #1: 1-fluoro-2-methyl-3-nitrobenzene With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.166667h; Stage #2: Dimethyl oxalate In N,N-dimethyl-formamide at 0 - 25℃; for 13h; Further stages.; | 80% |
-
-
123-75-1
pyrrolidine

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
344790-94-9
1-(2-(2-fluoro-6-nitrophenyl)vinyl)pyrrolidine

| Conditions | Yield |
|---|---|
| for 48h; Reflux; | 80% |
| In N,N-dimethyl-formamide at 40 - 100℃; for 1h; Inert atmosphere; |

-
-
123-75-1
pyrrolidine

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 3h; Heating; | 77% |


| Conditions | Yield |
|---|---|
| With paraformaldehyde In dimethyl sulfoxide; ethyl acetate; SiO2; tert-butyl alcohol | 71% |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
118665-03-5
2-(2-fluoro-6-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 70℃; for 1h; Inert atmosphere; | 69% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; dibenzoyl peroxide In tetrachloromethane at 90℃; for 14h; | 66% |
| 44% | |
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane Heating; | 22% |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
1644-82-2
2-fluoro-6-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1-fluoro-2-methyl-3-nitrobenzene; N,N-dimethyl-formamide dimethyl acetal at 135℃; for 12h; Stage #2: With sodium periodate In water; N,N-dimethyl-formamide at 20℃; for 3h; | 23% |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
95-92-1
oxalic acid diethyl ester

-
-
7593-91-1
3-(2-fluoro-6-nitrophenyl)-2-oxopropanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-fluoro-2-methyl-3-nitrobenzene; oxalic acid diethyl ester With ethanol; sodium for 0.75h; Reflux; Stage #2: With water In ethanol | 21% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(ll) chloride | |
| With hydrogenchloride; tin(ll) chloride | |
| With hydrogenchloride; tin(ll) chloride at 0 - 50℃; for 2h; |

| Conditions | Yield |
|---|---|
| With nitric acid | |
| With potassium permanganate | |
| Multi-step reaction with 3 steps 1: NBS, (PhCO)2O2 / CCl4 2: aq. Na2CO3 3: aq. H2SO4, K2Cr2O7 View Scheme | |
| With potassium permanganate In water | 11.81 g (40%) |
| With potassium permanganate; potassium carbonate In water |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
95-92-1
oxalic acid diethyl ester

-
-
399-68-8
4-fluoro-1H-indole-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; potassium ethoxide Behandeln des Reaktionsprodukts mit wss. NH3 unf FeSO4; | |
| Stage #1: 1-fluoro-2-methyl-3-nitrobenzene; oxalic acid diethyl ester With sodium ethanolate In diethyl ether at 35 - 38℃; for 18h; Stage #2: With ferrous(II) sulfate heptahydrate In aq. ammonia; water |
-
-
22630-08-6
tris(piperidino)-methane

-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
387-43-9
4-fluoroindole

| Conditions | Yield |
|---|---|
| With iron; silica gel 1) 120 deg C, 3 h, under vacuum 2) 1 h reflux in toluene-acetic acid; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium ethoxide In ethanol for 6h; Heating; | |
| Stage #1: oxalic acid diethyl ester With potassium tert-butylate In diethyl ether for 0.166667h; Stage #2: 1-fluoro-2-methyl-3-nitrobenzene In diethyl ether at 20℃; |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

-
-
912819-11-5
methyl 3-(2-fluoro-6-nitrophenyl)-2-oxobut-3-enoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: NaH / dimethylformamide; various solvent(s) / 0.17 h / 0 °C 1.2: 80 percent / dimethylformamide; various solvent(s) / 13 h / 0 - 25 °C 2.1: NaH / tetrahydrofuran; various solvent(s) / 1 h / 0 °C 2.2: 74 percent / tetrahydrofuran; various solvent(s) / 12 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 80 percent / sodium hydride / dimethylformamide / 19 h / 0 - 25 °C 2: 74 percent / sodium hydride / tetrahydrofuran / 13 h / 0 - 25 °C View Scheme |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaH / dimethylformamide; various solvent(s) / 0.17 h / 0 °C 1.2: 80 percent / dimethylformamide; various solvent(s) / 13 h / 0 - 25 °C 2.1: NaH / tetrahydrofuran; various solvent(s) / 1 h / 0 °C 2.2: 74 percent / tetrahydrofuran; various solvent(s) / 12 h / 25 °C 3.1: 61 percent / SnCl2*2H2O; molecular sieves 4 Angstroem / 1,2-dimethoxy-ethane / 1.5 h / 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / sodium hydride / dimethylformamide / 19 h / 0 - 25 °C 2: 74 percent / sodium hydride / tetrahydrofuran / 13 h / 0 - 25 °C 3: 61 percent / tin(II) chloride dihydrate; molecular sieves 4 Angstroem / 1,2-dimethoxy-ethane / 1.5 h / 40 °C View Scheme |
-
-
769-10-8
1-fluoro-2-methyl-3-nitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaH / dimethylformamide; various solvent(s) / 0.17 h / 0 °C 1.2: 80 percent / dimethylformamide; various solvent(s) / 13 h / 0 - 25 °C 2.1: NaH / tetrahydrofuran; various solvent(s) / 1 h / 0 °C 2.2: 74 percent / tetrahydrofuran; various solvent(s) / 12 h / 25 °C 3.1: 44 percent / SnCl2*2H2O; molecular sieves 4 Angstroem / 1,2-dimethoxy-ethane / 1.5 h / 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / sodium hydride / dimethylformamide / 19 h / 0 - 25 °C 2: 74 percent / sodium hydride / tetrahydrofuran / 13 h / 0 - 25 °C 3: 44 percent / tin(II) chloride dihydrate; molecular sieves 4 Angstroem / 1,2-dimethoxy-ethane / 1.5 h / 40 °C View Scheme |
2-Fluoro-6-nitrotoluene Specification
The 2-Fluoro-6-nitrotoluene, with the CAS registry number 769-10-8, is also known as Benzene, 1-fluoro-2-methyl-3-nitro-. It belongs to the product categories of Fluorides; Fluorin-Contained Toluene Series; Aromatic Hydrocarbons (substituted) & Derivatives; Halogen Toluene; Nitro Compounds; Nitrogen Compounds; Organic Building Blocks; Fluorinated Benzene Series. Its EINECS registry number is 212-203-3. This chemical's molecular formula is C7H6FNO2 and molecular weight is 155.13. What's more, both its IUPAC name and systematic name are the same which is called 1-Fluoro-2-methyl-3-nitrobenzene.
Physical properties about 2-Fluoro-6-nitrotoluene are: (1)ACD/LogP: 2.36; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.36; (4)ACD/LogD (pH 7.4): 2.36; (5)ACD/BCF (pH 5.5): 36.61; (6)ACD/BCF (pH 7.4): 36.61; (7)ACD/KOC (pH 5.5): 458.02; (8)ACD/KOC (pH 7.4): 458.02; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 45.82 Å2; (13)Index of Refraction: 1.53; (14)Molar Refractivity: 37.61 cm3; (15)Molar Volume: 121.7 cm3; (16)Surface Tension: 40.6 dyne/cm; (17)Density: 1.274 g/cm3; (18)Flash Point: 88.9 °C; (19)Enthalpy of Vaporization: 44.21 kJ/mol; (20)Boiling Point: 224.3 °C at 760 mmHg; (21)Vapour Pressure: 0.137 mmHg at 25 °C.
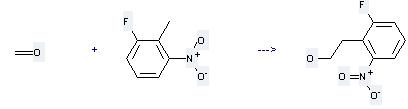
Uses of 2-Fluoro-6-nitrotoluene: it is used to produce other chemicals. For example, it can react with Formaldehyde to get 2-(2-Fluoro-6-nitrophenyl)ethanol. This reaction needs reagent potassium hydroxide and solvent N,N-dimethyl-acetamide at temperature of 20 °C.
When you are dealing with this chemical, you should be very careful. It is harmful by inhalation, in contact with skin and if swallowed. This chemical is irritating to eyes, respiratory system and skin. Therefore, you should avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: Fc1cccc([N+]([O-])=O)c1C
(2) InChI: InChI=1S/C7H6FNO2/c1-5-6(8)3-2-4-7(5)9(10)11/h2-4H,1H3
(3) InChIKey: GXPIVRKDWZKIKZ-UHFFFAOYSA-N
Related Products
- 2-Fluoro-6-nitrotoluene
- 769109-13-9
- 769-11-9
- 769123-68-4
- 769124-05-2
- 7691-28-3
- 769140-74-1
- 769158-12-5
- 76918-62-2
- 769195-26-8
- 769-19-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View