-
Name
3,4-(Methylenedioxy)cinnamic acid
- EINECS 219-151-0
- CAS No. 2373-80-0
- Article Data106
- CAS DataBase
- Density 1.41 g/cm3
- Solubility
- Melting Point 242-244 °C (dec.)(lit.)
- Formula C10H8O4
- Boiling Point 361.5oC at 760 mmHg
- Molecular Weight 192.171
- Flash Point 148.9oC
- Transport Information
- Appearance white to light yellow granular powder
- Safety 36-24/25
- Risk Codes 38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cinnamicacid, 3,4-(methylenedioxy)- (6CI,7CI,8CI);3,4-(Methylenedioxy)benzene-3-acrylic acid;3-(1,3-Benzodioxol-5-yl)-2-propenoic acid;3-(1,3-Benzodioxol-5-yl)acrylicacid;3-(3,4-Methylenedioxyphenyl)propenoic acid;3-(Benzodioxol-5-yl)acrylicacid;3',4'-Methylenedioxycinnamic acid;Cinnamic acid,3,4-[methylenebis(oxy)]-;NSC 5953;Piperonylideneacetic acid;
- PSA 55.76000
- LogP 1.51310
Synthetic route
-
-
40918-96-5
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate With water; sodium hydroxide In tetrahydrofuran Stage #2: With hydrogenchloride In water pH=< 3; | 99% |
| With sodium hydroxide In tetrahydrofuran; methanol at 40℃; Inert atmosphere; | 81% |
| With potassium hydroxide In ethanol; water Heating; | 15 mg |

| Conditions | Yield |
|---|---|
| With aluminum oxide; lithium chloride for 0.1h; Doebner condensation; microwave irradiation; | 98% |
| With piperidine; pyridine for 1h; Knoevenagel-Doebner-Stobbe Reaction; Reflux; | 98% |
| With ammonium acetate for 0.0666667h; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: piperonal; acetic acid With titanium tetrachloride In dichloromethane at 25℃; for 0.333333h; Inert atmosphere; Stage #2: With triethylamine In dichloromethane at 25℃; Inert atmosphere; stereoselective reaction; | 98% |

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With zinc In acetic acid at 20℃; for 0.0166667h; microwave irradiation; | 96% |
-
-
85580-21-8
3,4-methylenedioxycinnamic acid benzhydryl ester

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With formic acid at 40 - 45℃; for 0.5h; Product distribution; | 89% |
-
-
16669-99-1
(E)-2'-hydroxy-3,4-methylenedioxychalcone

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In acetonitrile at 20℃; for 5h; | 89% |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
A
-
7315-32-4
5-vinyl-1,3-benzodioxole

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With pyridine; acetic acid at 130℃; for 0.133333h; Knoevenagel-Doebner reaction; microwave irradiation; | A 4 % Spectr. B 85% |


| Conditions | Yield |
|---|---|
| With potassium acetate for 6h; Heating; | 50% |
| With sodium acetate |
-
-
117824-56-3
wikstromol

-
A
-
118975-42-1
4-Benzenesulfonylamino-2-benzo[1,3]dioxol-5-ylmethyl-4-oxo-3-(3,4,5-trimethoxy-benzyl)-butyric acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With n-butyllithium 1) -78 --> 0 deg C, several hours; 2) -78 --> RT; Yield given. Multistep reaction; | A n/a B 30% |

-
-
120-57-0
piperonal

-
-
127-09-3
sodium acetate

-
-
108-24-7
acetic anhydride

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
64-19-7
acetic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


| Conditions | Yield |
|---|---|
| With sodium Behandeln mit methylalkoholischer Kalilauge; |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
124-40-3
dimethyl amine

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
24393-66-6
ethyl (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With water; potassium hydroxide In tetrahydrofuran |
-
-
25173-72-2
3-(3,4,5-trimethoxyphenyl)propanoic acid

-
A
-
139747-16-3
2-piperonyl-3-(3,4,5-trimethoxy-benzyl)-succinic acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide Yield given; |

-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
7664-41-7
ammonia

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
64-17-5
ethanol

-
-
7722-84-1
dihydrogen peroxide

-
-
69662-23-3
4-benzo[1,3]dioxol-5-yl-2-oxo-but-3-enoic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With ethanol; dihydrogen peroxide |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
7664-41-7
ammonia

-
A
-
4436-15-1
piperonylidene-malonic acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 304 mg / conc. H2SO4 / 1 h / Heating 2: K2CO3, Cu0 / dimethylformamide / 1 h / Heating 3: 15 mg / KOH / ethanol; H2O / Heating View Scheme |
-
-
3843-74-1, 67667-67-8
Methyl caffeate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3, Cu0 / dimethylformamide / 1 h / Heating 2: 15 mg / KOH / ethanol; H2O / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 110 °C / Inert atmosphere 2: sodium hydroxide / methanol; tetrahydrofuran / 40 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) LDA, 2.) CBr4 / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 2 h 2: 92 percent / KI / acetone / 24 h / Heating 3: NaH / 0 °C 4: 1.) LDA View Scheme | |
| Multi-step reaction with 4 steps 1: 1.) LDA, 2.) CBr4 / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 2 h 2: 92 percent / KI / acetone / 24 h / Heating 3: NaH / 0 °C 4: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) LDA, 2.) iodine / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 10 min 2: NaH / 0 °C 3: 1.) LDA View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) LDA, 2.) iodine / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 10 min 2: NaH / 0 °C 3: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |
-
-
56183-75-6
3-(3,4-methylenedioxyphenyl)-2-bromopropionic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / KI / acetone / 24 h / Heating 2: NaH / 0 °C 3: 1.) LDA View Scheme | |
| Multi-step reaction with 3 steps 1: 92 percent / KI / acetone / 24 h / Heating 2: NaH / 0 °C 3: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |
-
-
118975-38-5
3-Benzo[1,3]dioxol-5-yl-2-iodo-propionic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaH / 0 °C 2: 1.) LDA View Scheme | |
| Multi-step reaction with 2 steps 1: NaH / 0 °C 2: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; piperidine 2: ethanolic KOH-solution View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / tetrahydrofuran / 0 °C 2: potassium hydroxide; water / tetrahydrofuran View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / 60 °C 2: pyridine; piperidine / 24 h / 80 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetone / 29 h / 50 - 60 °C 2: pyridine; piperidine / 24 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: caesium carbonate / N,N-dimethyl-formamide / 24 h / 100 °C 2.1: pyridine / 0.17 h / 20 °C 2.2: 4 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / Reflux 2: pyridine; piperidine / 24 h / 80 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydrogencarbonate / N,N-dimethyl-formamide / 12 h / 80 °C 2: acetic anhydride / isopropyl alcohol / 1 h / 55 - 110 °C 3: piperidine; pyridine / 2 h / 115 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydrogencarbonate / N,N-dimethyl-formamide / 12 h / 80 °C 2.1: acetic anhydride / isopropyl alcohol / 0.5 h / 55 °C 2.2: 1 h / 110 °C 2.3: 3 h 3.1: pyridine; piperidine / 2 h / 115 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic anhydride / isopropyl alcohol / 1 h / 55 - 110 °C 2: piperidine; pyridine / 2 h / 115 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: acetic anhydride / isopropyl alcohol / 0.5 h / 55 °C 1.2: 1 h / 110 °C 1.3: 3 h 2.1: pyridine; piperidine / 2 h / 115 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In N,N-dimethyl-formamide under 760 Torr; for 24h; | 100% |
| With palladium 10% on activated carbon; hydrogen; acetic acid In methanol at 20℃; for 22h; | 99% |
| With 10% Pd/C; cyclohexa-1,4-diene In methanol at 100℃; for 0.0833333h; Microwave irradiation; | 95% |
-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
96249-87-5
(E)-3-(1,3-benzodioxol-5-yl)acryloyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride at 25℃; for 0.5h; | 100% |
| With oxalyl dichloride at 25℃; for 2h; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 0 - 20℃; | 100% |
-
-
67-56-1
methanol

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
40918-96-5
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate

| Conditions | Yield |
|---|---|
| With sulfuric acid Heating; | 99% |
| With sulfuric acid for 24h; Reflux; | 98% |
| With sulfuric acid for 0.166667h; Esterification; Irradiation; | 96% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
40918-96-5
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether at 20℃; for 0.25h; Inert atmosphere; | 99% |
| 2 g | |
| In methanol; diethyl ether at 0℃; for 0.666667h; Esterification; |
-
-
88-04-0
4-Chloro-3,5-dimethylphenol

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
850496-03-6
4-(benzo[d][1,3]dioxol-5-yl)-6-chloro-5,7-dimethyl-3,4-dihydrochromen-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 23h; | 99% |
| With trifluoroacetic acid |
-
-
40918-96-5
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate With water; sodium hydroxide In tetrahydrofuran Stage #2: With hydrogenchloride In water pH=< 3; | 99% |
| With sodium hydroxide In tetrahydrofuran; methanol at 40℃; Inert atmosphere; | 81% |
| With potassium hydroxide In ethanol; water Heating; | 15 mg |

| Conditions | Yield |
|---|---|
| With aluminum oxide; lithium chloride for 0.1h; Doebner condensation; microwave irradiation; | 98% |
| With piperidine; pyridine for 1h; Knoevenagel-Doebner-Stobbe Reaction; Reflux; | 98% |
| With ammonium acetate for 0.0666667h; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: piperonal; acetic acid With titanium tetrachloride In dichloromethane at 25℃; for 0.333333h; Inert atmosphere; Stage #2: With triethylamine In dichloromethane at 25℃; Inert atmosphere; stereoselective reaction; | 98% |

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With zinc In acetic acid at 20℃; for 0.0166667h; microwave irradiation; | 96% |
-
-
85580-21-8
3,4-methylenedioxycinnamic acid benzhydryl ester

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With formic acid at 40 - 45℃; for 0.5h; Product distribution; | 89% |
-
-
16669-99-1
(E)-2'-hydroxy-3,4-methylenedioxychalcone

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In acetonitrile at 20℃; for 5h; | 89% |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
A
-
7315-32-4
5-vinyl-1,3-benzodioxole

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With pyridine; acetic acid at 130℃; for 0.133333h; Knoevenagel-Doebner reaction; microwave irradiation; | A 4 % Spectr. B 85% |


| Conditions | Yield |
|---|---|
| With potassium acetate for 6h; Heating; | 50% |
| With sodium acetate |
-
-
117824-56-3
wikstromol

-
A
-
118975-42-1
4-Benzenesulfonylamino-2-benzo[1,3]dioxol-5-ylmethyl-4-oxo-3-(3,4,5-trimethoxy-benzyl)-butyric acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With n-butyllithium 1) -78 --> 0 deg C, several hours; 2) -78 --> RT; Yield given. Multistep reaction; | A n/a B 30% |

-
-
120-57-0
piperonal

-
-
127-09-3
sodium acetate

-
-
108-24-7
acetic anhydride

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
64-19-7
acetic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


| Conditions | Yield |
|---|---|
| With sodium Behandeln mit methylalkoholischer Kalilauge; |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
124-40-3
dimethyl amine

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

-
-
24393-66-6
ethyl (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With water; potassium hydroxide In tetrahydrofuran |
-
-
25173-72-2
3-(3,4,5-trimethoxyphenyl)propanoic acid

-
A
-
139747-16-3
2-piperonyl-3-(3,4,5-trimethoxy-benzyl)-succinic acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide Yield given; |

-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
7664-41-7
ammonia

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
64-17-5
ethanol

-
-
7722-84-1
dihydrogen peroxide

-
-
69662-23-3
4-benzo[1,3]dioxol-5-yl-2-oxo-but-3-enoic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With ethanol; dihydrogen peroxide |
-
-
120-57-0
piperonal

-
-
141-82-2
malonic acid

-
-
7664-41-7
ammonia

-
A
-
4436-15-1
piperonylidene-malonic acid

-
B
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 304 mg / conc. H2SO4 / 1 h / Heating 2: K2CO3, Cu0 / dimethylformamide / 1 h / Heating 3: 15 mg / KOH / ethanol; H2O / Heating View Scheme |
-
-
3843-74-1, 67667-67-8
Methyl caffeate

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3, Cu0 / dimethylformamide / 1 h / Heating 2: 15 mg / KOH / ethanol; H2O / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 110 °C / Inert atmosphere 2: sodium hydroxide / methanol; tetrahydrofuran / 40 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) LDA, 2.) CBr4 / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 2 h 2: 92 percent / KI / acetone / 24 h / Heating 3: NaH / 0 °C 4: 1.) LDA View Scheme | |
| Multi-step reaction with 4 steps 1: 1.) LDA, 2.) CBr4 / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 2 h 2: 92 percent / KI / acetone / 24 h / Heating 3: NaH / 0 °C 4: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) LDA, 2.) iodine / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 10 min 2: NaH / 0 °C 3: 1.) LDA View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) LDA, 2.) iodine / 1) THF, -78 deg C (45 min), 0 deg C (3 1/4 h), RT (1 h); 2) THF, -78 deg C, 10 min 2: NaH / 0 °C 3: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |
-
-
56183-75-6
3-(3,4-methylenedioxyphenyl)-2-bromopropionic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / KI / acetone / 24 h / Heating 2: NaH / 0 °C 3: 1.) LDA View Scheme | |
| Multi-step reaction with 3 steps 1: 92 percent / KI / acetone / 24 h / Heating 2: NaH / 0 °C 3: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |
-
-
118975-38-5
3-Benzo[1,3]dioxol-5-yl-2-iodo-propionic acid

-
-
2373-80-0
3,4-methylenedioxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaH / 0 °C 2: 1.) LDA View Scheme | |
| Multi-step reaction with 2 steps 1: NaH / 0 °C 2: 30 percent / 1.) n-BuLi / 1) -78 --> 0 deg C, several hours; 2) -78 --> RT View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; piperidine 2: ethanolic KOH-solution View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / tetrahydrofuran / 0 °C 2: potassium hydroxide; water / tetrahydrofuran View Scheme |
3,4-(Methylenedioxy)cinnamic acid Chemical Properties
Molecular structure of (CAS NO.2373-80-0) is:
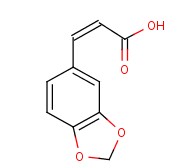
Product Name: trans-3,4-(Methylenedioxy)cinnamic acid
CAS Registry Number: 2373-80-0
IUPAC Name: (Z)-3-(1,3-benzodioxol-5-yl)prop-2-enoic acid
Molecular Weight: 192.16812 [g/mol]
Molecular Formula: C10H8O4
XLogP3: 2.1
H-Bond Donor: 1
H-Bond Acceptor: 4
EINECS: 219-151-0
Melting Point: 242-244 °C (dec.)(lit.)
Surface Tension: 65.4 dyne/cm
Density: 1.41 g/cm3
Flash Point: 148.9 °C
Enthalpy of Vaporization: 64.08 kJ/mol
Boiling Point: 361.5 °C at 760 mmHg
Vapour Pressure: 7.39E-06 mmHg at 25°C
Product Categories: Aromatic Cinnamic Acids, Esters and Derivatives;Cinnamic acid;C10;Carbonyl Compounds;Carboxylic Acids
3,4-(Methylenedioxy)cinnamic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 38
R38:Irritating to skin.
Safety Statements: 36-24/25
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
HazardClass: IRRITANT
3,4-(Methylenedioxy)cinnamic acid Specification
trans-3,4-(Methylenedioxy)cinnamic acid , its cas register number is 2373-80-0. It also can be called 2-Propenoic acid,3-(1,3-benzodioxol-5-yl)- ; 2-Propenoic acid, 3-(1,3-benzodioxol-5-yl)- ; 3-(3,4-Methylenedioxyphenyl)propenoic acid ; 3,4-(Methylenedioxy)benzene-3-acrylic acid ; 3,4-(Methylenedioxy)cinnamic acid ; 3-Benzo[1,3]dioxol-5-ylacrylic acid .It is a white to light yellow granular powder.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 237385-15-8
- 2373-98-0
- 2374-03-0
- 2374-05-2
- 237405-39-9
- 237407-59-9
- 237413-05-7
- 2374-14-3
- 2374-22-3
- 237424-17-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View