-
Name
4-Amino-2,6-dichloropyridine
- EINECS -0
- CAS No. 2587-02-2
- Article Data22
- CAS DataBase
- Density 1.497 g/cm3
- Solubility
- Melting Point 169-173 °C(lit.)
- Formula C5H4Cl2N2
- Boiling Point 336.7 °C at 760 mmHg
- Molecular Weight 163.006
- Flash Point 157.4 °C
- Transport Information
- Appearance white crystals
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Pyridine,4-amino-2,6-dichloro- (6CI,7CI,8CI);(2,6-Dichloropyridin-4-yl)amine;2,6-Dichloro-4-aminopyridine;2,6-Dichloropyridin-4-amine;NSC 136573;
- PSA 38.91000
- LogP 2.55180
Synthetic route
-
-
25194-01-8
2,6-dichloro-4-nitro-pyridine

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iron In ethanol; water at 95℃; for 16h; | 95.1% |
| With hydrogenchloride; water; iron In ethanol at 95℃; for 16h; | 95.1% |
| With ammonium hydroxide In tetrahydrofuran at 95℃; for 4h; | 82% |
-
-
5398-44-7
2,6-dichloropyridine-4-carboxylic acid

-
-
76-05-1
trifluoroacetic acid

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloropyridine-4-carboxylic acid; trifluoroacetic acid With sodium azide; oxalyl dichloride In dichloromethane Curtius Rearrangement; Heating / reflux; Stage #2: With potassium carbonate In methanol | 78% |
-
-
16063-69-7
2,4,6-trichloropyridine

-
A
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
B
-
116632-24-7
2-amino-4,6-dichloropyridine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In water at 130℃; for 4h; | A n/a B 67% |
| With ammonium hydroxide at 160℃; |

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloropyridine With 2,2,6,6-tetramethylpiperidinylmagnesium chloride lithium chloride complex Inert atmosphere; Stage #2: With C10H17NO In tetrahydrofuran; toluene at -25℃; for 2h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; water; toluene Inert atmosphere; | 64% |
| Multi-step reaction with 3 steps 1: CF3COOH; aq. H2O2 / 6.5 h / Heating 2: HNO3; H2SO4 / 160 °C 3: AcOH; Fe / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate; trifluoroacetic acid; dihydrogen peroxide / chloroform; water 2: nitric acid; sulfuric acid 3: hydrogenchloride / ethanol; water View Scheme |
-
-
2402-78-0
2,6-dichloropyridine

-
A
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
B
-
53344-76-6
2,2',6,6'-tetrachloro-4,4'-bipyridine

| Conditions | Yield |
|---|---|
| With potassium permanganate; ammonia; potassium amide 1.) DME, -70 deg C, 2.) DME, -70 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol for 8h; | 5.93 g |
-
-
42521-08-4
2,6-dichloroisonicotinoyl chloride

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloroisonicotinoyl chloride With sodium azide In water; acetone Stage #2: With trifluoroacetic acid In benzene for 16h; Heating; Stage #3: With potassium carbonate In methanol for 8h; | 5.93 g |
| Multi-step reaction with 3 steps 1: sodium azide / water; acetone / 0.03 h / 25 °C 2: trifluoroacetic acid / benzene / 21 h / 90 °C 3: potassium carbonate / methanol / 7.5 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HNO3; H2SO4 / 160 °C 2: AcOH; Fe / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid; sulfuric acid 2: hydrogenchloride / ethanol; water View Scheme | |
| Multi-step reaction with 2 steps 1.1: nitric acid; sulfuric acid / water / 1 h / 148 - 156 °C 1.2: pH 6 2.1: hydrogenchloride; iron / ethanol; water / 16 h / 95 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: nitric acid; sulfuric acid / 1 h / 148 - 156 °C 1.2: 20 °C / pH 6 2.1: hydrogenchloride; water; iron / ethanol / 16 h / 95 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; potassium nitrate / 7 h / 20 - 100 °C 2: acetic acid; iron / 3 h / 45 °C View Scheme |
-
-
42521-09-5
methyl 2,6-dichloroisonicotinate

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: lithium hydroxide monohydrate / tetrahydrofuran; water / 0.33 h / 25 °C 2: oxalyl dichloride / dichloromethane; tetrahydrofuran / 4 h / 25 °C 3: sodium azide / water; acetone / 0.03 h / 25 °C 4: trifluoroacetic acid / benzene / 21 h / 90 °C 5: potassium carbonate / methanol / 7.5 h / 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrazine hydrate / ethanol / 50 - 55 °C 2: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 3: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 4: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrazine hydrate / ethanol / 50 - 55 °C 2: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 3: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 4: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: trichlorophosphate / 24 h / 130 °C 2: lithium hydroxide monohydrate / tetrahydrofuran; water / 0.33 h / 25 °C 3: oxalyl dichloride / dichloromethane; tetrahydrofuran / 4 h / 25 °C 4: sodium azide / water; acetone / 0.03 h / 25 °C 5: trifluoroacetic acid / benzene / 21 h / 90 °C 6: potassium carbonate / methanol / 7.5 h / 25 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: trichlorophosphate / 40 - 105 °C 1.2: 20 - 60 °C 2.1: hydrazine hydrate / ethanol / 50 - 55 °C 3.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 4.1: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 5.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: trichlorophosphate / 40 - 105 °C 1.2: 20 - 60 °C 2.1: hydrazine hydrate / ethanol / 50 - 55 °C 3.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 4.1: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 5.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: trichlorophosphate / 40 - 105 °C 2.1: thionyl chloride / dichloromethane / 0 - 45 °C 2.2: 0 - 10 °C 3.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 4.1: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 5.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: trichlorophosphate / 40 - 105 °C 2.1: thionyl chloride / dichloromethane / 0 - 45 °C 2.2: 0 - 10 °C 3.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 4.1: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 5.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 4 h / 25 °C 2: sodium azide / water; acetone / 0.03 h / 25 °C 3: trifluoroacetic acid / benzene / 21 h / 90 °C 4: potassium carbonate / methanol / 7.5 h / 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: thionyl chloride / dichloromethane / 0 - 45 °C 1.2: 0 - 10 °C 2.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 3.1: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 4.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: thionyl chloride / dichloromethane / 0 - 45 °C 1.2: 0 - 10 °C 2.1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 3.1: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 4.1: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme |
-
-
81001-09-4
2,6-dichloropyridine-4-carbonylazide

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic acid / benzene / 21 h / 90 °C 2: potassium carbonate / methanol / 7.5 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 2: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 2: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme |
-
-
159178-03-7
2,6-dichloro-4-isocyanatopyridine

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloro-4-isocyanatopyridine With potassium carbonate In methanol at 25℃; for 7.5h; Stage #2: With water | 4.11 g |
| With hydrogenchloride In water; toluene at 40 - 45℃; |
-
-
2587-01-1
2,6-dichloro-4-nitropyridine-1-oxide

-
-
25194-01-8
2,6-dichloro-4-nitro-pyridine

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With iron; acetic acid at 45℃; for 3h; | 1.3 g |
-
-
501907-61-5
tert-butyl (2,6-dichloropyridin-4-yl)carbamate

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; toluene at 40 - 45℃; |
-
-
57803-51-7
2,6-dichloro-isonicotinic acid hydrazide

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 2: sodium hydroxide / toluene; water / 60 - 70 °C / pH 7 - 8 3: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogenchloride; sodium nitrite / toluene; water / 0 - 10 °C 2: sodium hydroxide / toluene; water / 90 - 100 °C / pH 7 - 8 3: hydrogenchloride / toluene; water / 40 - 45 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 0℃; for 2h; | 100% |
| With sulfuric acid; nitric acid In water at -5 - 10℃; for 2.66667h; | 100% |
| With sulfuric acid; nitric acid at -5℃; | 100% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
79-04-9
chloroacetyl chloride

-
-
1352830-49-9
2-chloro-N-(2,6-dichloro-pyridin-4-yl)-acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; N,N-dimethyl acetamide at 100℃; for 7h; | 97% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine | 97% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1044148-88-0
tert-butyl N-tert-butoxycarbonyl-N-(2,6-dichloro-4-pyridyl)carbamate

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In tetrahydrofuran at 25℃; for 16h; | 96% |
| Stage #1: 2,6-dichloro-[4]pyridylamine With sodium hexamethyldisilazane In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran at 20℃; for 16h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloro-[4]pyridylamine With sulfuric acid at -5℃; Stage #2: With nitric acid In water at 0 - 80℃; Stage #3: With ammonia In water pH=~ 4; Cooling with acetone-dry ice; | 94% |
| With sulfuric acid; nitric acid at 0 - 20℃; for 1h; Inert atmosphere; | 80% |
| With sulfuric acid; nitric acid at 0 - 80℃; for 2h; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloro-[4]pyridylamine; benzaldehyde In toluene for 12h; Heating / reflux; Stage #2: With sodium tetrahydroborate In ethanol at 50 - 75℃; for 2h; | 94% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
15568-85-1
5-methoxymethylene-2,2-dimethyl-1,3-dioxane-4,6-dione

-
-
863785-65-3
5-[[(2,6-dichloropyridin-4-yl)amino]methylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 70℃; for 4h; | 94% |
| In isopropyl alcohol Heating; | 92% |
| In isopropyl alcohol at 110℃; for 3h; | |
| In isopropyl alcohol at 20 - 75℃; for 0.75h; |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 16h; | 93.8% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
501907-61-5
tert-butyl (2,6-dichloropyridin-4-yl)carbamate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; | 93% |
| With sodium hexamethyldisilazane In tetrahydrofuran at 29℃; for 3h; | |
| With sodium hexamethyldisilazane In tetrahydrofuran | |
| With dmap In tetrahydrofuran at 60 - 70℃; for 23h; | 2.04 g |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
124-41-4
sodium methylate

-
-
1008304-85-5
2-chloro-6-methoxy-pyridin-4-ylamine

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide In methanol at 100℃; for 7h; Reagent/catalyst; Solvent; Autoclave; | 91.9% |
| In 1-methyl-pyrrolidin-2-one at 140℃; | 74% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
98-80-6
phenylboronic acid

-
-
52816-93-0
4-amino-2,6-diphenylpyridine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene at 100℃; for 5h; Inert atmosphere; | 89% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
141-52-6
sodium ethanolate

-
-
904311-14-4
2-chloro-6-ethoxy-pyridin-4-amine

| Conditions | Yield |
|---|---|
| In ethanol at 150℃; for 6h; | 88% |
| In ethanol at 150℃; for 6h; Sealed tube; | 88% |
| In ethanol at 150℃; for 2h; |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
222297-34-9
ethyl 4-[(3,5-dichloro-pyridin-4-yl)carbamoyl]-7-methoxybenzofuran-2-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; thionyl chloride In tetrahydrofuran; dichloromethane | 88% |
-
-
110-91-8
morpholine

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
878809-77-9
2-chloro-6-(morpholin-4-yl)-pyridin-4-ylamine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 150℃; | 87.8% |
| at 150℃; for 4h; Sealed tube; | 85% |
| at 150℃; for 4h; Sealed tube; | 85% |
-
-
67-56-1
methanol

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
124-41-4
sodium methylate

-
-
1008304-85-5
2-chloro-6-methoxy-pyridin-4-ylamine

| Conditions | Yield |
|---|---|
| at 130 - 160℃; Inert atmosphere; | 87% |

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With thionyl chloride; dicyclohexyl-carbodiimide In sulfolane for 8h; Inert atmosphere; Autoclave; Reflux; | 86.4% |

| Conditions | Yield |
|---|---|
| In sulfolane at 150℃; | 85% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 85% |
-
-
67-56-1
methanol

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
1008304-85-5
2-chloro-6-methoxy-pyridin-4-ylamine

| Conditions | Yield |
|---|---|
| With sodium methylate for 72h; Reflux; | 81.2% |
| at 150℃; for 0.5h; Microwave irradiation; | 79% |
| With sodium In methanol at 150℃; for 0.5h; Microwave irradiation; | 79% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
34160-24-2
2,5-dimethoxytetrahydrofuran

-
-
805229-07-6
2,6-dichloro-4-(1H-pyrrol-1-yl)pyridine

| Conditions | Yield |
|---|---|
| With acetic acid at 90℃; for 2h; | 80% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
108-86-1
bromobenzene

-
-
929033-63-6
2,6-dichloro-N,N-diphenylpyridin-4-amine

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In toluene for 24h; Inert atmosphere; Reflux; | 78% |
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In toluene for 24h; Inert atmosphere; Reflux; | 15g |

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; | 77% |

| Conditions | Yield |
|---|---|
| at 140℃; for 4h; | 77% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloro-[4]pyridylamine With sodium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.5h; Stage #2: C15H11ClN2O In tetrahydrofuran at -78 - 20℃; for 2h; | 76% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
1380809-63-1
4-chloro-5,6,7,8-tetrahydrobenzo[1,2-b]pyrimidino[5,4-d]selenophene

-
-
1380808-60-5
(2,6-dichloropyridin-4-yl)-5,6,7,8-tetrahydrobenzo[1,2-b]pyrimidino[5,4-d]selenophen-4-ylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 20℃; for 16h; | 73% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; tert-butyl XPhos In toluene at 20 - 110℃; Inert atmosphere; | 72% |
-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
3282-30-2
pivaloyl chloride

-
-
1345456-45-2
N-(2,6-dichloro-4-pyridinyl)-2,2-dimethylpropanamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 13h; | 70% |
| With triethylamine In dichloromethane Cooling with ice; | |
| With triethylamine In dichloromethane Cooling with ice; | 11.59 g |
-
-
622-40-2
2-(morpholin-4-yl)ethanol

-
-
2587-02-2
2,6-dichloro-[4]pyridylamine

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
1432740-81-2
2-(morpholin-4-yl)ethyl (2,6-dichloropyridin-4-yl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dichloro-[4]pyridylamine; bis(trichloromethyl) carbonate With triethylamine In benzene for 5h; Reflux; Stage #2: 2-(morpholin-4-yl)ethanol In benzene at 20℃; | 70% |

4-Amino-2,6-dichloropyridine Chemical Properties
IUPAC Name: 2,6-Dichloropyridin-4-amine
Synonyms: 2,6-Dichloropyridin-4-amine ; 4-Pyridinamine, 2,6-dichloro- ; 2,6-Dichloro-4-aminopyridine ; 2,6-Dichloro-4-pyridinamine ; 2,6-Dichloropyridin-4-ylamine
Product Categories: Pyridine;Nucleotides and Nucleosides;Bases & Related Reagents;Nucleotides
Molecular Structure of 4-Amino-2,6-dichloropyridine (CAS NO.2587-02-2) : 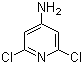
Molecular Formula of 4-Amino-2,6-dichloropyridine (CAS NO.2587-02-2) : C5H4Cl2N2
Molecular Weight of 4-Amino-2,6-dichloropyridine (CAS NO.2587-02-2) : 163.01
CAS NO: 2587-02-2
Index of Refraction: 1.622
Surface Tension: 56.9 dyne/cm
Density: 1.497 g/cm3
Flash Point: 157.4 °C
Enthalpy of Vaporization: 57.99 kJ/mol
Boiling Point: 336.7 °C at 760 mmHg
Vapour Pressure: 0.00011 mmHg at 25°C
Melting point 167-170 ºC
4-Amino-2,6-dichloropyridine Production
Raw materials :Etanol-->Hydrochloric acid-->Ethyl acetate-->Diethyl ether-->Sulfuric acid -->Acetic acid glacial-->Nitric acid-->Potassium carbonate-->Sodium carbonate-->Chloroform-->Sodium sulfate-->Acetone-->Hydrogen peroxide-->Iron-->Trifluoroacetic acid-->Phosphorus trichloride-->2,6-Dichloropyridine-->Kieserite
4-Amino-2,6-dichloropyridine Safety Profile
Hazard Codes  Xi
Xi
Risk Statements 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany 3
Hazard Note Irritant
Related Products
- 4-amino-2- butoxy-5-nitro-6-hydroxypyrimidine
- 4-Amino-2-(3-pyridyl)pyrimidine-5-carbonitrile
- 4-Amino-2-(4-methyl-1-piperazinyl)-5-pyrimidinecarbonitrile
- 4-Amino-2-(4-morpholinyl)-5-pyrimidinecarbonitrile
- 4-Amino-2-(methylthio)-6-pyrimidinol
- 4-Amino-2-(methylthio)pyrimidine-5-carboxaldehyde
- 4-Amino-2,2,5,5-tetrakis(trifluoro methyl)-3-imidazoline
- 4-Amino-2,2-dimethyl-butyric acid
- 4-Amino-2,3,5,6-tetrafluoropyridine
- 4-Amino-2,3-difluoro-5-nitrobenzoic acid
- 25870-62-6
- 25870-68-2
- 25870-73-9
- 25871-69-6
- 2587-42-0
- 25875-50-7
- 25875-51-8
- 25875-99-4
- 25876-45-3
- 25877-30-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View