-
Name
5-Chlorouracil
- EINECS 217-339-7
- CAS No. 1820-81-1
- Article Data26
- CAS DataBase
- Density 1.61 g/cm3
-
Solubility
slightly soluble in water
- Melting Point >300 °C(lit.)
- Formula C4H3ClN2O2
- Boiling Point 413.6 °C at 760 mmHg
- Molecular Weight 146.533
- Flash Point 203.9 °C
- Transport Information
- Appearance white to light beige crystalline powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
- Molecular Structure
- Hazard Symbols
 Xi
Xi
- Synonyms 2,4 (1H,3H)-Pyrimidinedione, 5-chloro-;2,4(1H,3H)-Pyrimidinedione, 5-chloro-;Uracil, 5-chloro- (VAN) (8CI);Uracil, 5-chloro-;5-Chloro-2,4-pyrimidinedione;5-Chlorouracil,5-Chloro-2,4-dihydroxypyrimidine;
- PSA 65.72000
- LogP -0.28340
Synthetic route
Conditions
Conditions Yield With 1,2-Dichloro-3-iodobenzene In water; acetic acid at 80℃; for 0.25h; 92% With hydrogenchloride; ammonium cerium(IV) nitrate In methanol at 70℃; for 5.5h; 82% With sodium hydrogencarbonate; lithium chloride In acetonitrile Ambient temperature; electrolysis (Pt electrodes, SCE reference, LiClO4 as supporting electrolyte); 77% With potassium phosphate; potassium chloride; dihydrogen peroxide; chloroperoxidase pH 3; 7% -
-
123551-49-5
5-chloro-2,4-dimethoxypyrimidine

-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride 69% 
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride; chlorine In water for 16h; Heating; 63% Conditions
Conditions Yield With sodium persulfate; water; sodium chloride In acetic acid at 100 - 105℃; A 20%
B 53%With sodium persulfate; water; sodium chloride In acetic acid at 100 - 105℃; A 20%
B 53%-
-
65906-87-8
5-chloro-6-hydroxy-5-methoxycarbonyl-5,6-dihydrouracil

-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride In water for 15h; Heating; 27% In hydrogenchloride -
-
128-09-6
N-chloro-succinimide

-
-
108-24-7
acetic anhydride

-
-
64-19-7
acetic acid

-
-
66-22-8
uracil

-
-
1820-81-1
5-chlorouracil
...Expand
-
-
106146-79-6
2-ethylmercapto-5-chloro-3H-pyrimidin-4-one

-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride 
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride -
-
40338-28-1
1-acetyl-1H-pyrimidine-2,4-dione

-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield Yield given. Multistep reaction; 
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydroxylamine at 25℃; Equilibrium constant; var. pH; 
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride; tin 
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride; tin Conditions
Conditions Yield With chlorine Conditions
Conditions Yield With phosphate buffer; thymidine phosphorylase from Escherichia coli at 37℃; pH=7; Enzyme kinetics; Further Variations:; concentration of reagent; -
-
42821-92-1
methyl 2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate

-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With hydrogenchloride In water -
-
1820-81-1
5-chlorouracil

-
-
65094-55-5
C4H4ClN2O2
Conditions
Conditions Yield With H2SO4 glasses Product distribution; Irradiation; 100% -
-
75-77-4
chloro-trimethyl-silane

-
-
1820-81-1
5-chlorouracil

-
-
58990-53-7
5-chloro-2,4-bis-O-trimethylsilyluracil
Conditions
Conditions Yield With 1,1,1,3,3,3-hexamethyl-disilazane Ambient temperature; 99% -
-
1820-81-1
5-chlorouracil

-
-
930-69-8
sodium thiophenolate

-
-
1137-90-2
5-(phenylsulfanyl)-2,4(1H,3H)-pyrimidinedione
Conditions
Conditions Yield In N,N,N,N,N,N-hexamethylphosphoric triamide at 150℃; for 0.0833333h; microwave irradiation; 97% -
-
1820-81-1
5-chlorouracil

-
-
140-88-5
ethyl acrylate

-
-
1370413-72-1
3-(5-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)propionic acid ethyl ester
Conditions
Conditions Yield With triethylamine In N,N-dimethyl-formamide at 60℃; Aza-Micheal reaction; 96% Conditions
Conditions Yield With phosphorus pentachloride; trichlorophosphate Neat (no solvent); Heating / reflux; 95% With phosgene; Triphenylphosphine oxide In nitrobenzene at -5 - 125℃; for 0.833333h; Inert atmosphere; 93% With N,N-diethylaniline; trichlorophosphate at 110℃; for 24h; 81% -
-
1820-81-1
5-chlorouracil

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
58990-53-7
5-chloro-2,4-bis-O-trimethylsilyluracil
Conditions
Conditions Yield With ammonium sulfate for 5h; Heating; 95% With chloro-trimethyl-silane Heating; With ammonium sulfate for 3h; Heating; -
-
1820-81-1
5-chlorouracil

-
-
292638-85-8
acrylic acid methyl ester

-
-
1256346-76-5
3-(5-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)propionic acid methyl ester
Conditions
Conditions Yield With triethylamine In N,N-dimethyl-formamide at 60℃; for 4h; Michael Addition; 95% With triethylamine In N,N-dimethyl-formamide at 20℃; Michael-type addition; regioselective reaction; 88% -
-
1820-81-1
5-chlorouracil

-
-
141-32-2
acrylic acid n-butyl ester

-
-
1370413-75-4
3-(5-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)propionic acid butyl ester
Conditions
Conditions Yield With triethylamine In N,N-dimethyl-formamide at 60℃; Aza-Micheal reaction; 95% Conditions
Conditions Yield With zinc(II) oxide at 130℃; for 0.1h; Ionic liquid; Microwave irradiation; 92% -
-
1820-81-1
5-chlorouracil

-
-
63331-62-4
5-Chloro-4-thiouracil
Conditions
Conditions Yield With diphosphorus pentasulfide; sodium hydrogencarbonate In diethylene glycol dimethyl ether at 110℃; for 3h; 90% -
-
1820-81-1
5-chlorouracil

-
-
604-68-2
α-D-glucopyranose peracetylate

-
-
379218-39-0
5-chloro-1-(2,3,4,6-tetra-O-acetyl-β-D-gluco-hexopyranosyl)uracil
Conditions
Conditions Yield Stage #1: 5-chlorouracil With 1,1,1,3,3,3-hexamethyl-disilazane; saccharin In acetonitrile for 3h; Heating;
Stage #2: α-D-glucopyranose peracetylate With trimethylsilyl trifluoromethanesulfonate In acetonitrile at 20 - 85℃;90% -
-
1820-81-1
5-chlorouracil

-
-
100-46-9
benzylamine

-
-
28485-19-0
5-(benzylamino)dihydropyrimidine-2,4(1H,3H)-dione
Conditions
Conditions Yield at 150℃; for 0.333333h; microwave irradiation; 88% Conditions
Conditions Yield In acetonitrile at 20℃; 87% Conditions
Conditions Yield In ethanol; chloroform; water for 8h; Reflux; 84% -
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield In methanol; dichloromethane (N2); using Schlenk techniques; addn. of suspn. of 5-chlorouracil in methanol to soln. of Zn(HB(Me3CC3HN2CHMe2)3)(OH) in CH2Cl2; stirring overnight; filtration, evapn. of solvent, drying under vac., elem. anal.; 81% -
-
1820-81-1
5-chlorouracil

-
-
36288-37-6
N-hydroxy-4-methylbenzenecarboximidoyl chloride
Conditions
Conditions Yield With dmap In 1,4-dioxane for 5h; Reflux; 80% -
-
1820-81-1
5-chlorouracil

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
121654-00-0
3,4,6-tris-O-tert-butyldimethylsilyl-1,2-anhydro-α-D-glucopyranose
Conditions
Conditions Yield Stage #1: 5-chlorouracil With 1,1,1,3,3,3-hexamethyl-disilazane at 120℃; for 1.5h; Inert atmosphere;
Stage #2: trimethylsilyl trifluoromethanesulfonate; 3,4,6-tris-O-tert-butyldimethylsilyl-1,2-anhydro-α-D-glucopyranose at 60℃; for 3h; Inert atmosphere;80% ...Expand
-
-
1820-81-1
5-chlorouracil

-
-
61946-91-6
4-ethyl-N-hydroxybenzene-1-carboximidoyl chloride
Conditions
Conditions Yield With dmap In 1,4-dioxane for 5h; Reflux; 79% -
-
1820-81-1
5-chlorouracil

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
74372-90-0, 112289-38-0, 135202-48-1, 148888-66-8, 71696-32-7
1,2-anhydro-3,4,6-tri-O-benzyl-β-D-mannopyranose
Conditions
Conditions Yield Stage #1: 5-chlorouracil With 1,1,1,3,3,3-hexamethyl-disilazane at 120℃; for 1.5h; Inert atmosphere;
Stage #2: trimethylsilyl trifluoromethanesulfonate; 1,2-anhydro-3,4,6-tri-O-benzyl-β-D-mannopyranose at 60℃; for 3h; Inert atmosphere;79% ...Expand
-
-
848644-37-1
1,2,3,5-tetra-O-acetyl-3-C-ethynyl-D-ribo-pentofuranose

-
-
1820-81-1
5-chlorouracil

-
-
848644-41-7
1-[2,3,5-tri-O-acetyl-3-C-ethynyl-β-D-ribo-pentofuranosyl]-5-chlorouracil
Conditions
Conditions Yield With N,O-bis-(trimethylsilyl)-acetamide; trimethylsilyl trifluoromethanesulfonate In acetonitrile at 50℃; for 29h; 78% Conditions
Conditions Yield With triethylamine In water at 100℃; for 0.0833333h; Michael addition; microwave irradiation; 78% Conditions
Conditions Yield With N-ethyl-N,N-diisopropylamine In methanol at 42℃; for 2h; 77% -
-
1820-81-1
5-chlorouracil

-
-
4998-57-6
His

-
-
203308-39-8
Cr(3+)*C4H2N2O2Cl(1-)*C6H8N3O2(1-)*H2O*Cl(1-) = Cr(C4H2N2O2Cl)(C6H8N3O2)(H2O)Cl
Conditions
Conditions Yield With triethyl formate; aq. NaOH In ethanol; water addn. aq. histidine to pptd. (pH 5-6, aq. NaOH) suspn. of metal/5-chlorouracil complex in ethanol; pptn. on concn. and ether addn., drying (vac., 50°C); elem. anal.; 75% ...Expand Conditions
Conditions
Conditions Yield With sodium hydroxide In water at 100℃; for 0.166667h; Microwave irradiation; regioselective reaction; 75% -
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With [D]-sodium hydroxide; sodium In water-d2 at 0 - 120℃; Inert atmosphere; 75% Conditions
Conditions Yield Stage #1: 5-chlorouracil With potassium carbonate In dimethyl sulfoxide at 80℃; for 0.5h;
Stage #2: 4-Fluoronitrobenzene In dimethyl sulfoxide at 70 - 80℃;74% -
-
1820-81-1
5-chlorouracil

-
-
108-98-5
thiophenol

-
-
1137-90-2
5-(phenylsulfanyl)-2,4(1H,3H)-pyrimidinedione
Conditions
Conditions Yield With potassium carbonate In ethylene glycol at 130 - 140℃; for 2h; 71% 
-
-
1820-81-1
5-chlorouracil

-
-
4998-57-6
His

-
-
203308-40-1
Fe(3+)*C4H2N2O2Cl(1-)*C6H8N3O2(1-)*H2O*Cl(1-) = Fe(C4H2N2O2Cl)(C6H8N3O2)(H2O)Cl
Conditions
Conditions Yield With triethyl formate; aq. NaOH In ethanol; water addn. aq. histidine to pptd. (pH 5-6, aq. NaOH) suspn. of metal/5-chlorouracil complex in ethanol; pptn. on concn. and ether addn., drying (vac., 50°C); elem. anal.; 70% ...Expand
-
-
1820-81-1
5-chlorouracil
Conditions
Conditions Yield With triethyl orthoformate In ethanol refluxing (2-3 h); pptn. (pH 5-6, aq. NaOH), washing (ethanol), drying (vac., 50°C);elem. anal.; 70% 5-Chlorouracil Specification
The 5-Chlorouracil, with the CAS registry number 1820-81-1 and EINECS registry number 217-339-7, has the IUPAC name of 5-chloro-1H-pyrimidine-2,4-dione. It is a kind of white to light beige crystalline powder, and belongs to the following product categories: Pyridines, Pyrimidines, Purines and Pteredines; Pyrimidine series; Heterocyclic Compounds; Biochemistry; Nucleobases and their analogs; Nucleosides, Nucleotides & Related Reagents; Nucleic acids; Bases & Related Reagents; Heterocycles; Nucleotides. The molecular formula of the chemical is C4H3ClN2O2. What's more, it is always used for the experimental and clinical treatment of neoplastic and viral diseases.
The physical properties of 5-Chlorouracil are as followings: (1)ACD/LogP: -0.52; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.52; (4)ACD/LogD (pH 7.4): -0.78; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 12.35; (8)ACD/KOC (pH 7.4): 6.76; (9)#H bond acceptors: 4; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 40.62 Å2; (13)Index of Refraction: 1.587; (14)Molar Refractivity: 30.56 cm3; (15)Molar Volume: 90.9 cm3; (16)Polarizability: 12.11×10-24cm3; (17)Surface Tension: 57.3 dyne/cm; (18)Density: 1.61 g/cm3; (19)Flash Point: 203.9 °C; (20)Enthalpy of Vaporization: 69.22 kJ/mol; (21)Boiling Point: 413.6 °C at 760 mmHg; (22)Vapour Pressure: 1.98E-07 mmHg at 25°C.
Uses of 5-Chlorouracil: It can react with chloro-trimethyl-silane to produce 5-Chloro-2,4-bis(trimethylsiloxy)pyrimidine. This reaction will need reagent hexamethylsilazane, and the yield is about 99%.
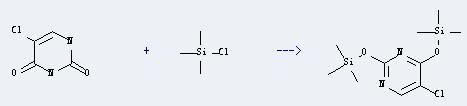
You should be cautious while dealing with this chemical. It irritates to eyes, respiratory system and skin. Therefore, you had better take the following instructions: Do not breathe dust and avoid contacting with skin and eyes; Wear suitable protective clothing, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Cl/C1=C/NC(=O)NC1=O
(2)InChI: InChI=1/C4H3ClN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
(3)InChIKey: ZFTBZKVVGZNMJR-UHFFFAOYASRelated Products
- 5-Chlorouracil
- 18209-34-2
- 18209-36-4
- 18209-60-4
- 18209-61-5
- 18209-66-0
- 1821-12-1
- 18212-20-9
- 18212-21-0
- 18212-22-1
- 1821-27-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View