-
Name
8-Hydroxyquinaldine
- EINECS 212-562-6
- CAS No. 826-81-3
- Article Data36
- CAS DataBase
- Density 1.21 g/cm3
- Solubility insoluble
- Melting Point 71-73 °C(lit.)
- Formula C10H9NO
- Boiling Point 267 °C at 760 mmHg
- Molecular Weight 159.188
- Flash Point 139.4 °C
- Transport Information UN 3077 9/PG 3
- Appearance beige to brown crystalline powder
- Safety 60-61-24/25-36-26
- Risk Codes 50/53-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, N
N
- Synonyms 2-Methyl-8-hydroxyquinoline;2-Methyl-8-quinolinol;2-Methyloxine;Hydroxyquinaldine;
- PSA 33.12000
- LogP 2.24880
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium iodide at 110℃; for 1h; | 95% |
-
-
61047-43-6
8-bromo-2-methylquinoline

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; N1-(4-hydroxy-2,6-dimethylphenyl)-N2-(4-hydroxy-3,5-dimethylphenyl)oxalamide; water In water; dimethyl sulfoxide at 80℃; for 24h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With 2-hydroxynitrobenzene In sulfuric acid; acetic acid at 120℃; for 8h; Heating; | 94% |
| With sulfuric acid; water; 2-hydroxynitrobenzene oder in wss. Salzsaeure; | |
| With hydrogenchloride; arsenic(V) oxide; water |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; iron(III) chloride hexahydrate at 150℃; for 8h; Inert atmosphere; Sealed tube; Autoclave; | 88% |

| Conditions | Yield |
|---|---|
| With phospho-tungstic acid; phosphotungstic acid; silica gel for 0.2h; Doebner-Miller reaction; microwave irradiation; | 85% |
| With hydrogenchloride at 90℃; for 5h; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 130℃; for 0.666667h; Friedlaender Quinoline Synthesis; Microwave irradiation; Inert atmosphere; | 84% |

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-hydroxyphenyl)butan-2-one O-2,4-dinitrophenyloxime With sodium hydride In 1,4-dioxane at 50℃; for 20h; Cyclization; Stage #2: With acetic acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,4-dioxane for 2h; Oxidation; Heating; | 80% |
| With sodium hydride; acetic acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone 1) dioxane, reflux, 0.5 h, 2) dioxane, reflux, 2 h; Yield given. Multistep reaction; |

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-hydroxyphenyl)butan-2-one (E)-O-2,4-dinitrophenyloxime With sodium hydride In 1,4-dioxane at 50℃; for 20h; Cyclization; Stage #2: With acetic acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,4-dioxane for 2h; Oxidation; Heating; | 80% |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; N1-(4-hydroxy-2,6-dimethylphenyl)-N2-(4-hydroxy-3,5-dimethylphenyl)oxalamide; water In water; dimethyl sulfoxide at 130℃; for 24h; Schlenk technique; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 36h; Inert atmosphere; Reflux; | 76% |

| Conditions | Yield |
|---|---|
| With palladium hydroxide 10 wt. % on activated carbon; hydrogen; triethylamine In ethanol at 43℃; under 9750.98 - 11251.1 Torr; for 6h; Solvent; Reagent/catalyst; Pressure; | 62.67% |

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; oxygen; palladium diacetate; trifluoroacetic acid at 150℃; for 36h; Schlenk technique; | 58% |
-
-
81485-78-1
8-hydroxy-2-methyl-1,2,3,4-tetrahydroquinoline

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium tert-butylate; oxygen; nickel dibromide In tert-Amyl alcohol at 95℃; for 48h; | 56% |
-
-
3033-80-5
8-methoxy-2-methylquinoline

-
-
74-89-5
methylamine

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
128278-09-1
8-methylaminoquinaldine

| Conditions | Yield |
|---|---|
| With ammonium chloride In ethanol at 200℃; for 20h; | A 30% B 55% |

-
-
3033-80-5
8-methoxy-2-methylquinoline

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
128278-09-1
8-methylaminoquinaldine

| Conditions | Yield |
|---|---|
| With ammonium chloride; methylamine In ethanol at 200℃; for 20h; | A 30% B 55% |
-
-
1004545-76-9
2-amino-3-(1-hydroxybut-3-enyl)phenol

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; air; palladium diacetate In methanol at 40℃; under 760.051 Torr; for 36h; | 53% |

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
81485-78-1
8-hydroxy-2-methyl-1,2,3,4-tetrahydroquinoline

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,4-dioxane at 50℃; for 20h; Cyclization; | A 36% B 41% |

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
81485-78-1
8-hydroxy-2-methyl-1,2,3,4-tetrahydroquinoline

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,4-dioxane for 0.5h; Heating; | A 37% B 33% |
| With sodium hydride In 1,4-dioxane for 0.5h; Product distribution; Heating; var. of base, no differences in reactions of the isolated (E)- and (Z)-isomers; | A 37% B 33% |

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,4-dioxane for 0.5h; Heating; | A 20% B 7% |
| With air; sodium hydride In 1,4-dioxane at 50℃; for 20h; Cyclization; | A 20% B 7% |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; aniline hydrochloride |
-
-
849585-22-4
LACTIC ACID

-
-
95-55-6
2-amino-phenol

-
-
88-75-5
2-hydroxynitrobenzene

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With sulfuric acid |


| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With hydrogenchloride; aluminium trichloride |

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With potassium hydroxide |
-
-
108348-16-9
tetrakis(2-methyl-8-quinolinolato)thorium(IV) * 2-methyl-8-quinolinol

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| 70-155°C; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In neat (no solvent) VO(meox)2-O-VO(meox)2 heated in Abderhalden's dryer with sodium hydroxide as a trap for vaporized 8-quinolinol; complex recrystd. from chlorobenzene; |
-
-
213881-38-0
2-methyl-8-benzenesulfonyloxyquinoline

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
3198-32-1
benzenesulfonate

| Conditions | Yield |
|---|---|
| In water-d2; [D3]acetonitrile at 25℃; pH=7.4; Quantum yield; HEPES buffer; UV-irradiation; Inert atmosphere; |
-
-
1419402-08-6
2-methyl-8-quinolinyl-β-D-glucopyranoside

-
A
-
826-81-3
2-methyl-8-quinolinol

-
B
-
492-61-5
β-D-glucose

| Conditions | Yield |
|---|---|
| With β-glucosidase from almonds; water In aq. phosphate buffer at 37℃; for 4h; pH=7.4; Reagent/catalyst; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid; sodium iodide / 6 h / 110 °C / Inert atmosphere 2: hydrogen bromide / 36 h / Inert atmosphere; Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With Aeromonas proteolytica aminopeptidase; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid In dimethyl sulfoxide at 25℃; pH=8; Kinetics; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| for 15h; Acetylation; Heating; | 100% |
| Reflux; | 99% |
| at 138℃; for 5h; | 95% |
-
-
899797-70-7
2-(3-bromomethyl-2,4-dichlorobenzenesulfonylamino)-2-methylpropionic acid tert-butyl ester

-
-
826-81-3
2-methyl-8-quinolinol

-
-
635697-85-7
2-[2,4-dichloro-3-(2-methylquinolin-8-yloxymethyl)benzenesulfonylamino]-2-methylpropionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-8-quinolinol With sodium hydride In N,N-dimethyl-formamide Stage #2: 2-(3-bromomethyl-2,4-dichlorobenzenesulfonylamino)-2-methylpropionic acid tert-butyl ester In N,N-dimethyl-formamide at 20℃; | 100% |

-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| In ethanol room temp., equiv. amts., pptn.; | 100% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
107-30-2
chloromethyl methyl ether

-
-
1262838-22-1
8-(methoxymethoxy)-2-methylquinoline

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-8-quinolinol With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; Stage #2: chloromethyl methyl ether In N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; | 100% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
98-09-9
benzenesulfonyl chloride

-
-
213881-38-0
2-methyl-8-benzenesulfonyloxyquinoline

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 1.5h; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 24.5h; | 97% |
| With triethylamine In benzene at 20 - 100℃; | 91% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
256652-07-0
2-methyl-8-quinolinyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 3h; triflation; | 99% |
| With pyridine In dichloromethane at 0 - 25℃; Inert atmosphere; | 98% |
| With 2,6-dimethylpyridine In dichloromethane at -20℃; for 1h; | 98% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
50-00-0
formaldehyd

-
-
23978-55-4
1,4,10,13-tetraoxa-7,16-diazacyclooctadecane

-
-
1245934-87-5
1,10-bis((2-methyl-8-hydroxy-7-quinolinyl)methyl)-1,10-diaza-18-crown-6 ether

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Product distribution / selectivity; Microwave irradiation; | 99% |
| In 1,4-dioxane for 2h; Mannich reaction; Inert atmosphere; Microwave irradiation; | 95% |


| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; N-chloro-succinimide In dichloromethane at 35℃; for 8h; Temperature; Solvent; Darkness; Green chemistry; | 98.8% |
| With hydrogenchloride; chlorine In water Reagent/catalyst; Inert atmosphere; Darkness; | 98.9% |
| With hydrogenchloride; sodium hypochlorite In water at 20 - 30℃; for 4.5h; Temperature; | 97.7% |

| Conditions | Yield |
|---|---|
| In toluene N2-atmosphere; stirring (room temp., 5 min); pptn. on concg. (vac., -40°C, 24 h), filtering; | 98.5% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
1412254-56-8
8-(cyclopropylmethoxy)-2-methylquinoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 88℃; for 48h; Sealed flask; | 98.5% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 13h; | |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 13h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 10h; | 98% |
| With sodium carbonate In ethanol; acetone at 20℃; for 12h; Darkness; | 90% |
| With sodium carbonate In ethanol; acetone at 20℃; for 12h; Inert atmosphere; | 90% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
15599-52-7
5,7-dibromo-2-methylquinolin-8-ol

| Conditions | Yield |
|---|---|
| With bromine In methanol at 20℃; for 0.0833333h; | 98% |
| With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; | 97% |
| With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; | 97% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
171735-72-1
8-tert-butyldimethylsilyloxy-2-methyl-quinoline

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 20℃; | 98% |
| With 1H-imidazole In dichloromethane at 20℃; for 10h; | 96% |
| With 1H-imidazole In dichloromethane | |
| In dichloromethane | |
| With 1H-imidazole In dichloromethane at 20℃; for 12h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With acetic acid In toluene for 0.25h; Inert atmosphere; Glovebox; Schlenk technique; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-8-quinolinol With potassium tert-butylate In tetrahydrofuran for 3h; Reflux; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 6h; Inert atmosphere; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 97% |
| Stage #1: 2-methyl-8-quinolinol With triethylamine In acetone at 20℃; for 0.333333h; Stage #2: methyl iodide In acetone at 20℃; for 8h; Darkness; | 95% |

| Conditions | Yield |
|---|---|
| In nitrobenzene at 175 - 180℃; for 3h; | 97% |

| Conditions | Yield |
|---|---|
| In toluene to a toluene soln. of AlEt3 was added dropwise a toluene soln. of 2-methyl-8-quinolinol for 1 h, the mixt. was stirred at room temp. for 12 h (inert atm.); ppt. was washed with toluene and dried in vac.; elem. anal.; | 97% |
| In toluene byproducts: C2H6; (N2); Al(C2H5)3 in toluene added dropwise to a soln. of ligand, stirred for 12 h at 298 K; sepd., evapd. (vac.), washed (hexane), filtered, dried (vac.); elem. anal.; | 64% |
| In toluene Heating / reflux; |

| Conditions | Yield |
|---|---|
| In toluene (inert gas); reaction of aluminium compd. with quinolinol deriv. in ratio 1:2 in toluene; I. Yamaguchi, T. Iijima, T. Yamamoto, J. Orgamonet. Chem. 654 (2002) 229 (Erratum: J.Organomet. Chem. 658 (2002) 281); NMR; | 97% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 5.5h; | 97% |
-
-
826-81-3
2-methyl-8-quinolinol

| Conditions | Yield |
|---|---|
| With water-d2; benzoic acid at 120℃; for 4h; | 97% |
| With water-d2; benzoic acid at 120℃; for 8h; Inert atmosphere; Schlenk technique; |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
694443-67-9
2-[2-(3,4-dichlorophenyl)vinyl]quinoline-8-ol

| Conditions | Yield |
|---|---|
| In acetic anhydride at 120℃; for 20h; Inert atmosphere; | 96.9% |
| Multi-step reaction with 2 steps 1: 16 h / 130 °C / Inert atmosphere 2: pyridine; water / 3 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 20℃; for 2h; Irradiation; | 96% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
32247-96-4
3,5-bis(trifluoromethyl)benzyl bromide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In tetrahydrofuran at 20℃; | 96% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
18978-78-4
2-methyl-8-aminoquinoline

| Conditions | Yield |
|---|---|
| With ammonium sulfate; ammonium hydroxide at 170℃; for 48h; | 95% |
| With ammonium hydroxide; 2H3N*O3S(2-)*H2O*2H(1+) at 170℃; for 48h; | 45% |
| With ammonium hydroxide; sodium hydrogensulfite at 200℃; |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
14510-06-6
2-formyl oxine

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In 1,4-dioxane at 80℃; for 24h; | 95% |
| With selenium(IV) oxide In 1,4-dioxane; water at 80℃; for 24h; Inert atmosphere; | 90% |
| With selenium(IV) oxide In 1,4-dioxane; water at 60℃; for 12.5h; Reflux; | 86% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
32665-18-2
tetrakis(trimethylsilylmethyl)zirconium(IV)

| Conditions | Yield |
|---|---|
| In pentane; benzene N2-atmosphere; stirring (room temp., 1 h); evapn. (vac.); | 95% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
4923-85-7
tert-butylphenylphosphinic acid chloride

-
-
1227180-24-6
2-methylquinolin-8-yl tert-butyl(phenyl)posphinate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 24h; Reflux; Inert atmosphere; | 95% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
51608-60-7
phenyl N-tosyl imine

-
-
1218989-08-2
N-(2-(8-hydroxyquinolin-2-yl)-1-phenylethyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In tetrahydrofuran at 120℃; for 24h; Inert atmosphere; screw-cap vial; | 95% |
-
-
826-81-3
2-methyl-8-quinolinol

-
-
626-15-3
1,3-bis-(bromomethyl)benzene

-
-
1369787-70-1
1,3-bis-(2-methyl-8-quinolyloxymethyl)benzene

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 20℃; for 4h; | 95% |
| Stage #1: 2-methyl-8-quinolinol With potassium carbonate In acetonitrile at 80℃; for 0.166667h; Stage #2: 1,3-bis-(bromomethyl)benzene In acetonitrile at 80℃; for 12h; | 80% |
| Stage #1: 2-methyl-8-quinolinol With potassium carbonate In acetonitrile at 80℃; for 0.166667h; Stage #2: 1,3-bis-(bromomethyl)benzene In acetonitrile at 80℃; for 12h; | 80% |
8-Hydroxyquinaldine Consensus Reports
Reported in EPA TSCA Inventory.
8-Hydroxyquinaldine Specification
The CAS registry number of 8-Hydroxy-2-methylquinoline is 826-81-3. The IUPAC name is 2-methylquinolin-8-ol. Its EINECS registry number is 212-562-6. In addition, the molecular formula is C10H9NO and the molecular weight is 159.18. It is a kind of beige to brown crystalline powder and belongs to the classes of Quinolines, Quinazolines and derivatives; Alkylquinolines; Hydroxyquinolines; Quinolines.
Physical properties about this chemical are: (1)ACD/LogP: 2.33; (2)ACD/LogD (pH 5.5): 0.85; (3)ACD/LogD (pH 7.4): 0.93; (4)ACD/BCF (pH 5.5): 1.14; (5)ACD/BCF (pH 7.4): 1.39; (6)ACD/KOC (pH 5.5): 14.5; (7)ACD/KOC (pH 7.4): 17.61; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Polar Surface Area: 22.12 Å2; (12)Index of Refraction: 1.666; (13)Molar Refractivity: 48.89 cm3; (14)Molar Volume: 131.4 cm3; (15)Polarizability: 19.38 ×10-24cm3; (16)Surface Tension: 54.9 dyne/cm; (17)Density: 1.21 g/cm3; (18)Flash Point: 139.4 °C; (19)Enthalpy of Vaporization: 52.54 kJ/mol; (20)Boiling Point: 267 °C at 760 mmHg; (21)Vapour Pressure: 0.00508 mmHg at 25°C.
Preparation of 8-Hydroxy-2-methylquinoline: it can be prepared by o-aminophenol and crotonylal. Firstly, mix o-aminophenol with o-nitrophenol. Then add hydrochloric acid and crotonylal with stirring. Heat it for 6 hours and steam out the o-nitrophenol by steam distillation. You can get the crude by alkalization, neutralization and steam distillation. At last, you can obtain the product via a series of vacuum distillation, recrystallization and purification.
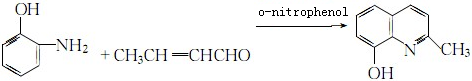
Uses of 8-Hydroxy-2-methylquinoline: it can be used as reagent for the analysis and extractant to determine manganese, palladium, gallium, indium, beryllium, etc. It also can be used as precipitant for the determination of zinc, magnesium, etc. In addition, it can be used to get 2-methyl-quinolin-8-ylamine. This reaction will need reagents (NH4)2SO4*H2O and NH3(aq.). The reaction time is 2 days at reaction temperature of 170 °C. The yield is about 95%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. And it is very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. During using it, wear suitable protective clothing and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. This material and its container must be disposed of as hazardous waste. In addition, you should avoid release to the environment and you can refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: Oc1cccc2ccc(nc12)C
(2)InChI: InChI=1/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3
(3)InChIKey: NBYLBWHHTUWMER-UHFFFAOYAF
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View