-
Name
Aluminum acetate
- EINECS 205-354-1
- CAS No. 139-12-8
- Article Data49
- CAS DataBase
- Density 1.002 g/cm3
- Solubility soluble H2O [HAW93]
- Melting Point decomposes with moisture [CRC10]
- Formula C6H9AlO6
- Boiling Point 117.1 °C at 760 mmHg
- Molecular Weight 204.115
- Flash Point 40 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Aceticacid, aluminum salt (8CI,9CI);Aluminum acetate (6CI);Aluminium acetate;Aluminum triacetate;Aluminum(III) acetate;Buro-SolConcentrate;Burow solution;Burow's solution;Domeboro;
- PSA 78.90000
- LogP 0.04140
Synthetic route

| Conditions | Yield |
|---|---|
| With aluminium trichloride Abdestillieren des gebildeten Acetylchlorids und Erhitzen des Rueckstands auf 160-180grad; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| at 90℃; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) symmetrization on heating;; |

| Conditions | Yield |
|---|---|
| In water purification from Pb ions with H2S, from SO4 ions with barium acetate; | |
| In water purification from Pb ions with H2S, from SO4 ions with barium acetate; |


| Conditions | Yield |
|---|---|
| With diethylsulfide In benzene byproducts: (C2H5)2SO2; addn. of t-BuOOH to soln. of (t-BuO)3Al and Et2S in benzene for 1-2 min at 20°C; condencing of solvent and volatile components with liq. N2; addn. of H2SO4 to residue, treatment with acetic acid; filtration, removal of volatiles under reduced pressure; | |
| With diisopropylsulfide In benzene byproducts: (isopropyl)2SO2; addn. of t-BuOOH to soln. of (t-BuO)3Al and i-Pr2S in benzene for 1-2 min at 20°C; condencing of solvent and volatile components with liq. N2; addn. of H2SO4 to residue, treatment with acetic acid; filtration, removal of volatiles under reduced pressure; | |
| With dimethylsulfide In benzene byproducts: (CH3)2SO2; addn. of t-BuOOH to soln. of (t-BuO)3Al and Me2S in benzene for 1-2 min at 20°C; condencing of solvent and volatile components with liq. N2; addn. of H2SO4 to residue, treatment with acetic acid; filtration, removal of volatiles under reduced pressure; |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In benzene byproducts: (isopropyl)2SO2, (CH3)3COH; (Ar); addn. of (CH3)3COOH (2 equiv.) to soln. of ((CH3)3CO)3Al (1 equiv.) and (isopropyl)2S (1 equiv.) in benzene at 20°C; self-heating; treatment with AcOH; filtration; removal of volatiles under reduced pressure; | |
| With tert.-butylhydroperoxide; diethyl sulphide In benzene byproducts: (C2H5)2SO2, (CH3)3COH; (Ar); addn. of (CH3)3COOH (2 equiv.) to soln. of ((CH3)3CO)3Al (1 equiv.) and (C2H5)2S (1 equiv.) in benzene at 20°C; self-heating; treatment with AcOH; filtration; removal of volatiles under reduced pressure; | |
| With tert.-butylhydroperoxide; dimethylsulfide In benzene byproducts: (CH3)2SO2, (CH3)3COH; (Ar); addn. of (CH3)3COOH (2 equiv.) to soln. of ((CH3)3CO)3Al (1 equiv.) and (CH3)2S (1 equiv.) in benzene at 20°C; self-heating; treatment with AcOH; filtration; removal of volatiles under reduced pressure; |

| Conditions | Yield |
|---|---|
| In water Electrochem. Process; electrolytical dissolution of Al in acetic acid; calcium acetate addition for increase of electric conductivity; | |
| dissolving Al in water free acetic acid; | |
| In water influence of concentration and temperature were examined; |
-
-
7446-70-0
aluminium trichloride

-
-
64-19-7
acetic acid

-
A
-
7647-01-0
hydrogenchloride

-
B
-
139-12-8
aluminum acetate

| Conditions | Yield |
|---|---|
| water free AlCl3, water free acetic acid; driving away HCl; inpurity less then 1 % Cl; |


| Conditions | Yield |
|---|---|
| With barium carbonate In water | |
| With calcium carbonate In water | |
| With BaCO3 In water | |
| With CaCO3 In water |

| Conditions | Yield |
|---|---|
| In water | |
| In water | |
| With acetic anhydride In acetic acid Al(OH)3 in 1:1-mixt. of glacial acetic acid and acetic anhydride refluxed for 24 h; suspn. filtered, solid washed with acetone; |

| Conditions | Yield |
|---|---|
| With hydroxide In acetic acid Al(OH)3 pptd. from soln. of aluminum ammonium sulfate; Al(OH)3 filtered out; quickly dissolved in glacial CH3COOH; |

| Conditions | Yield |
|---|---|
| In water equiv. amount of educts; | |
| In water equiv. amount of educts; |

| Conditions | Yield |
|---|---|
| In water | |
| In water |
-
-
7446-70-0
aluminium trichloride

-
-
108-24-7
acetic anhydride

-
A
-
139-12-8
aluminum acetate

-
B
-
75-36-5
acetyl chloride

| Conditions | Yield |
|---|---|
| distn. the formed acetyl chloride and heating the residue at 160-180 °C; | |
| distn. the formed acetyl chloride and heating the residue at 160-180 °C; |


| Conditions | Yield |
|---|---|
| In water purification from Pb ions with H2S, from SO4 ions with barium acetate; | |
| In water purification from Pb ions with H2S, from SO4 ions with barium acetate; |


| Conditions | Yield |
|---|---|
| In water | |
| In water |
-
-
555-75-9
aluminum ethoxide

-
-
108-24-7
acetic anhydride

-
A
-
139-12-8
aluminum acetate

-
B
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| heating at 90 °C and distn. the formed ethyl acetate; | |
| heating at 90 °C and distn. the formed ethyl acetate; |


| Conditions | Yield |
|---|---|
| heating in sealed tube; | |
| heating in sealed tube; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) symmetrization on heating;; |
-
-
591-87-7
Allyl acetate

-
-
3530-39-0
phenylaluminium dichloride

-
A
-
300-57-2
allylbenzene

-
B
-
92-52-4
biphenyl

-
C
-
139-12-8
aluminum acetate

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In diethyl ether Ar atmosphere; stirring (20°C, 5 mole-% catalyst, 20 h); | A 0% B <1 C n/a |


| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride With acetic acid at 110℃; for 0.5h; Stage #2: aluminum (III) chloride at 20℃; |

| Conditions | Yield |
|---|---|
| at 100℃; for 0.166667h; |

| Conditions | Yield |
|---|---|
| In aq. acetate buffer at 50℃; for 1h; pH=5; pH-value; Temperature; | 94.38% |

-
-
139-12-8
aluminum acetate

| Conditions | Yield |
|---|---|
| In pentan-1-ol mixt. of octakis(benzylthio)phthalocyaninato lithium and metal salt wererefluxed in n-pentanol for 1 h, cooled to room temp.; centrifuged, washed with hot EtOH, dried in desiccator; | 69% |

| Conditions | Yield |
|---|---|
| at 320℃; |


| Conditions | Yield |
|---|---|
| Stage #1: zinc diacetate; aluminum acetate With potassium hydroxide In water at 20℃; pH=14; Acidic conditions; Stage #2: With acetic acid In water pH=8; |

| Conditions | Yield |
|---|---|
| With acetic anhydride In acetic acid |

| Conditions | Yield |
|---|---|
| slow reaction, 90°C; | |
| slow reaction, 90°C; |

-
-
139-12-8
aluminum acetate

| Conditions | Yield |
|---|---|
| With ammonium fluoride; manganese(II) acetate In water to anodic aluminum hydroxyde added satd. solns. of ammonium fluoride, aluminum acetate, and manganese acetate; mixt. heated at 1000-1200°C for 0.5-5 h; elem. anal.; |

| Conditions | Yield |
|---|---|
| Al salt introduced into SiO2 sol, hydrolyzed, pptd. by (CH2)6N4, heatedat 1200-1250°C for 1 h; not isolated, detected by XRD; |


| Conditions | Yield |
|---|---|
| In water pptn. and washing with ethanol; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) mixt. heated at 950°C for 1 h, mixt. ground up in a mortar, fired at 900°C for 1 h in air; not isolated, mixt. of phases: NaAlO2 and γ-Al2O3; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) mixt. heated at 950°C for 10 h, mixt. ground up in a mortar, fired at 900°C for 1 h in air; not isolated, mixt. of phases: NaAlO2, β-Al2O3 and γ-Al2O3; |

Aluminum Acetate Chemical Properties
IUPAC Name: Aluminum triacetate
Synonyms: Acetic acid, aluminum salt (3:1) ; Aluminum acetate ; Aluminum acetate (USP) ; Aluminum acetate, basic ; Aluminum triacetate ; Basic aluminum acetate
Product Categories: Organic-metal salt
Molecular Structure of Aluminum Acetate (CAS NO.139-12-8) : 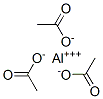
Molecular Formula of Aluminum Acetate (CAS NO.139-12-8) : C6H9AlO6
Molecular Weight of Aluminum Acetate (CAS NO.139-12-8) : 204.11
CAS NO : 139-12-8
EINECS : 205-354-1
Mol File: 139-12-8.mol
Flash Point: 40 °C
Enthalpy of Vaporization: 23.7 kJ/mol
Boiling Point: 117.1 °C at 760 mmHg
Vapour Pressure: 13.9 mmHg at 25°C
Aluminum Acetate Uses
1. Aluminum Acetate (CAS NO.139-12-8) is used for the mordant,materials of carmine pigment dyeing and printing .
2. Aluminum Acetate (CAS NO.139-12-8) is used for waterproofing agent and shampoo.
Related Products
- Aluminum 1,4,8,11,15,18,22,25-octabutoxy-29H,31H-phthalocyanine triethylsiloxide
- Aluminum aceglutamide
- Aluminum Acetate
- Aluminum acetylacetonate
- Aluminum ammonium sulfate
- Aluminum arsenide(AlAs)
- ALUMINUM AZIDE
- Aluminum barium magnesium oxide
- Aluminum barium titanium oxide
- Aluminum bismuth oxide(Al3BiO6), monohydrate (9CI)
- 1391-36-2
- 13913-82-1
- 139-13-9
- 139139-86-9
- 139139-93-8
- 1391-41-9
- 139143-09-2
- 139152-08-2
- 139162-43-9
- 139163-43-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View