-
Name
Benzyl carbamate
- EINECS 210-710-4
- CAS No. 621-84-1
- Article Data95
- CAS DataBase
- Density 1.168 g/cm3
- Solubility 68.02g/L(37 oC)
- Melting Point 86-89 °C(lit.)
- Formula C8H9NO2
- Boiling Point 318.723 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 182.584 °C
- Transport Information
- Appearance Off-white to light beige powder or flakes
- Safety 22-24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Carbamicacid, benzyl ester (6CI,7CI,8CI);Benzyl aminoformate;Carbamyl benzyl ester;NSC 25317;O-Benzylcarbamate;
- PSA 52.32000
- LogP 1.98220
Synthetic route
-
-
621-84-1
O-benzyl carbamate

-
-
35660-91-4, 35845-66-0, 495-41-0
(E)-1-phenyl-2-buten-1-one

| Conditions | Yield |
|---|---|
| With gold(III) chloride In dichloromethane at 20℃; for 2h; Product distribution; Further Variations:; Reagents; Aza-Michael reaction; | 100% |
| iridium tetrachloride for 2h; Product distribution / selectivity; Aza-Michael reaction; | 100% |
| osmium (III) chloride for 6h; Product distribution / selectivity; Aza-Michael reaction; | 96% |
-
-
621-84-1
O-benzyl carbamate

-
-
563-96-2
2,2-dihydroxyacetic acid

-
-
79002-45-2
N-benzyloxycarbonyl-glycine

| Conditions | Yield |
|---|---|
| In toluene at 40℃; | 100% |
| In toluene at 40℃; for 3.5h; | 100% |
| In toluene at 40℃; for 3.5h; Large scale reaction; | 96% |
| In diethyl ether at 20℃; for 10h; |
-
-
621-84-1
O-benzyl carbamate

-
-
873-55-2
sodium benzenesulfonate

-
-
105-07-7
4-cyanobenzaldehyde

-
-
1220349-77-8
benzyl (4-cyanophenyl)(phenylsulfonyl)methylcarbamate

| Conditions | Yield |
|---|---|
| With formic acid In tetrahydrofuran; water at 22℃; Inert atmosphere; | 100% |

-
-
924-44-7
glyoxylic acid ethyl ester

-
-
621-84-1
O-benzyl carbamate

-
-
104473-51-0
ethyl 2-(((benzyloxy)carbonyl)amino)-2-hydroxyacetate

| Conditions | Yield |
|---|---|
| In ethyl acetate at 60℃; for 24h; | 100% |
| With acetic acid In ethyl acetate; toluene at 60℃; for 14h; Inert atmosphere; | 97% |
| With acetic acid In ethyl acetate at 60℃; for 14h; Inert atmosphere; | 81% |
-
-
924-44-7
glyoxylic acid ethyl ester

-
-
621-84-1
O-benzyl carbamate

-
-
134746-23-9
ethyl 2-(((benzyloxy)carbonyl)amino)-2-chloroacetate

| Conditions | Yield |
|---|---|
| With acetic acid; acetyl chloride In chloroform at 60℃; for 12h; Solvent; Inert atmosphere; | 100% |
| With acetic acid; acetyl chloride In chloroform at 60℃; for 12h; | 98% |
| With thionyl chloride In chloroform; toluene for 12h; Inert atmosphere; Schlenk technique; Reflux; | 93% |
-
-
621-84-1
O-benzyl carbamate

-
-
64805-08-9
ethyl glyoxalate hydrate

-
-
134746-23-9
ethyl 2-(((benzyloxy)carbonyl)amino)-2-chloroacetate

| Conditions | Yield |
|---|---|
| With thionyl chloride In chloroform; toluene at 60℃; for 6h; Reagent/catalyst; Inert atmosphere; | 100% |
| With acetic acid; acetyl chloride In chloroform at 60℃; for 12h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With acetic acid; acetyl chloride In chloroform at 60℃; for 16h; Inert atmosphere; | 100% |
-
-
621-84-1
O-benzyl carbamate

-
-
64805-08-9
ethyl glyoxalate hydrate

-
-
577973-96-7
N-Cbz-α-bromoglycine ethyl ester

| Conditions | Yield |
|---|---|
| With Acetyl bromide; acetic acid In chloroform; toluene at 20℃; for 6.5h; Reagent/catalyst; Inert atmosphere; | 100% |
-
-
621-84-1
O-benzyl carbamate

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
71150-68-0
benzyl N-(4-formylphenyl)carbamate

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In tetrahydrofuran at 45℃; for 19h; Arylation; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
61752-66-7
(E)-1-phenyl-2-penten-1-one

| Conditions | Yield |
|---|---|
| copper(II) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 3h; | 99% |
| With Nafion(R) SAC-13 In acetonitrile at 20℃; for 24h; | 99% |
| With toluene-4-sulfonic acid In acetonitrile at 20℃; for 72h; Product distribution; Further Variations:; Reagents; reaction times; aza-Michael addition; | 99% |
| With copper(II) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 3h; aza-Michael addition; | 99% |
| dichloro bis(acetonitrile) palladium(II) In dichloromethane at 20℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride In dichloromethane at 20℃; for 8h; | 99% |
| With aluminium(III) triflate In nitromethane at 50℃; for 0.166667h; Microwave irradiation; | 99% |
| With iron(III) chloride at 60℃; neat (no solvent); | 96% |
-
-
621-84-1
O-benzyl carbamate

-
-
70400-19-0
C9H8BrNO3

-
-
1355707-71-9
benzyl [1-(3-bromo-4-methoxyphenyl)-2,2-dichloro-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
37630-26-5, 4735-49-3
β-(1-naphthyl)nitroethylene

-
-
1355707-76-4
benzyl [2,2-dichloro-1-(naphth-1-yl)-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
5153-69-5, 706-08-1
4-fluoro-β-nitrostyrene

-
-
1355707-67-3
benzyl [2,2-dichloro-1-(4-fluorophenyl)-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
37888-03-2, 3156-35-2
3-chloroβ-nitrostyrene

-
-
1355707-63-9
benzyl [2,2-dichloro-1-(3-chlorophenyl)-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
65185-68-4
o-bromonitrostyrene

-
-
1355707-66-2
benzyl [1-(2-bromophenyl)-2,2-dichloro-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
22568-07-6, 3156-34-1
1-chloro-2-(2-nitrovinyl)benzene

-
-
1355707-61-7
benzyl [2,2-dichloro-1-(2-chlorophenyl)-2-nitroethyl]carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; potassium carbonate In dichloromethane at 20℃; for 8h; regioselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
4663-33-6, 62668-02-4, 62839-70-7, 116127-91-4, 132014-29-0, 136981-81-2, 148616-45-9
1,3-diphenyl-3-hydroxypropene

-
-
928765-35-9
(R/S)-N-benzyloxycarbonyl-1,3-diphenylprop-2-en-1-ylamine

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride In dichloromethane at 20℃; for 0.166667h; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
17488-65-2
4-phenylbut-3-en-2-ol

-
-
920285-74-1
(E)-4-phenyl-3-buten-2-amine N-benzyloxycarbonyl ester

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride In dichloromethane at 20℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| With triethylsilane; perrhenic acid anhydride In dichloromethane at 20℃; for 0.5h; chemoselective reaction; | 99% |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
621-84-1
O-benzyl carbamate

-
-
302569-84-2
N-benzyloxycarbonyloxy-3-phenylpropylamine

| Conditions | Yield |
|---|---|
| With triethylsilane; perrhenic acid anhydride In dichloromethane at 20℃; for 1.5h; chemoselective reaction; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
7745-93-9
2-bromo-4-nitrotoluene

-
-
875118-52-8
benzyl (2-methyl-5-nitrophenyl)carbamate

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); triisopropylsilanol; potassium hydroxide In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 99% |
-
-
621-84-1
O-benzyl carbamate

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
126535-86-2
ethyl 2-(((benzyloxy)carbonyl)amino)-3,3,3-trifluoro-2-hydroxypropanoate

| Conditions | Yield |
|---|---|
| In dichloromethane | 98% |
-
-
621-84-1
O-benzyl carbamate

-
-
104-87-0
4-methyl-benzaldehyde

-
-
762-04-9
phosphonic acid diethyl ester

-
-
129960-70-9
benzyl (diethoxyphosphoryl)(p-tolyl)methylcarbamate

| Conditions | Yield |
|---|---|
| With thionyl chloride; acetic acid 1) room temperature, 20 min, 2) reflux, 12 h; | 98% |
| With acetyl chloride for 6h; Ambient temperature; Yield given; |


| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; tributylphosphine In dichloromethane at 30℃; for 24h; aza-Michael reaction; | 98% |
| With bismuth(III) nitrate In dichloromethane at 20℃; | 94% |
| [Pd(MeCN)2(DPCBH)](OTf)2 at 20℃; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; tributylphosphine In dichloromethane at 30℃; for 24h; aza-Michael reaction; | 98% |
| With Nafion(R) SAC-13 In acetonitrile at 20℃; for 12h; | 78% |
-
-
621-84-1
O-benzyl carbamate

-
-
62668-02-4
(E)-1,3-diphenyl-2-propen-1-ol

-
-
928765-35-9
(R/S)-N-benzyloxycarbonyl-1,3-diphenylprop-2-en-1-ylamine

| Conditions | Yield |
|---|---|
| With C36H40O20S4 In water at 60℃; for 24h; | 98% |
| With potassium hexafluorophosphate; bismuth(lll) trifluoromethanesulfonate; calcium sulfate In 1,4-dioxane at 23 - 26℃; for 0.2h; | 97% |
| With sodium tetrachloroaurate(III) dihyrate In dichloromethane for 1h; Reflux; | 94% |
Benzyl carbamate Specification
The Benzyl carbamate, with the CAS registry number 621-84-1, is also known as Carbamyl benzyl ester. It belongs to the product categories of Heterocycles; Nitrogen Compounds; Organic Building Blocks; Protected Amines; Building Blocks; Chemical Synthesis; Nitrogen Compounds. Its EINECS number is 210-710-4. This chemical's molecular formula is C8H9NO2 and molecular weight is 151.16. What's more, its systematic name is Benzyl carbamate. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides. It is used as organic synthesis intermediates. This chemical is a carbamate which is often used an amine protecting group in organic synthesis. It is commonly used in peptide synthesis. It is used to protect amines from electrophiles.
Physical properties of Benzyl carbamate are: (1)ACD/LogP: 1.31; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.31; (4)ACD/LogD (pH 7.4): 1.31; (5)ACD/BCF (pH 5.5): 5.83; (6)ACD/BCF (pH 7.4): 5.83; (7)ACD/KOC (pH 5.5): 122.91; (8)ACD/KOC (pH 7.4): 122.91; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 52.32 Å2; (13)Index of Refraction: 1.548; (14)Molar Refractivity: 41.112 cm3; (15)Molar Volume: 129.41 cm3; (16)Polarizability: 16.298×10-24cm3; (17)Surface Tension: 46.3 dyne/cm; (18)Density: 1.168 g/cm3; (19)Flash Point: 182.584 °C; (20)Enthalpy of Vaporization: 56.025 kJ/mol; (21)Boiling Point: 318.723 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by carbonochloridic acid benzyl ester at the temperature of 0 °C. This reaction will need reagent conc. ammonium hydroxide. The yield is about 92%.

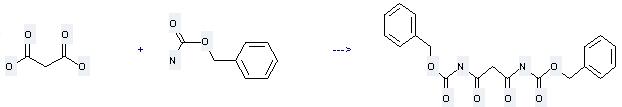
Uses of Benzyl carbamate: it can be used to produce Malonyl-di-benzylurethan at the temperature of 100 - 110 °C. It will need reagent acetic anhydride. The yield is about 84%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is harmful by inhalation, in contact with skin and if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing. You should not breathe dust. When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCc1ccccc1)N
(2)Std. InChI: InChI=1S/C8H9NO2/c9-8(10)11-6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,9,10)
(3)Std. InChIKey: PUJDIJCNWFYVJX-UHFFFAOYSA-N
Related Products
- Benzyl (1-(aminocarbonyl)-2-hydroxypropyl)carbamate
- Benzyl (1-cyano-1-methylethyl)carbamate
- Benzyl (1R,2S)-3-chloro-2-hydroxy-1-(phenylthiomethyl)propylcarbamate
- Benzyl (2,5-dioxo-1,3-oxazolidin-4-yl)acetate
- Benzyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (2R,3S)-(1-carbamoyl-2-hydroxypropyl)carbamate
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3R)-(+)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (3-aminopropyl)carbamate
- 621-87-4
- 621-88-5
- 621-91-0
- 621-95-4
- 6219-55-2
- 6219-71-2
- 6219-73-4
- 62197-94-8
- 62199-62-6
- 622-00-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View