-
Name
Cantharidin
- EINECS 200-263-3
- CAS No. 56-25-7
- Article Data12
- CAS DataBase
- Density 1.362 g/cm3
- Solubility 30mg/L(20 oC)
- Melting Point 215-217 °C(lit.)
- Formula C10H12O4
- Boiling Point 326.869 °C at 760 mmHg
- Molecular Weight 196.203
- Flash Point 146.137 °C
- Transport Information UN 2811 6.1/PG 1
- Appearance white to light yellow crystal powder
- Safety 53-45-36/37/39
- Risk Codes 28-36/37/38-38-37-36-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T,
T,  T+
T+
- Synonyms 4,7-Epoxyisobenzofuran-1,3-dione,hexahydro-3a,7a-dimethyl-, (3aa,4b,7b,7aa)-;7-Oxabicyclo[2.2.1]heptane-2,3-dicarboxylicanhydride, 2,3-dimethyl- (8CI);Cantharidin (6CI,7CI);1,2-Dimethyl-3,6-epoxyperhydrophthalic anhydride;Cantharidine;Cantharone;Hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione;Kantaridin;NSC 61805;
- PSA 52.60000
- LogP 0.64360
Synthetic route
-
-
110-00-9
furan

-
-
75532-25-1
2,2,4,4-tetrahydrothiophene-3,4-dicarboxylic acid anhydride

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Stage #1: furan; 2,2,4,4-tetrahydrothiophene-3,4-dicarboxylic acid anhydride With 1-butyl-3-methylimidazolium Tetrafluoroborate at 50℃; for 19h; Stage #2: In ethyl acetate for 4h; Reagent/catalyst; Temperature; Reflux; | 90% |
-
-
75538-64-6
C10H10O4S

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With hydrogen In ethyl acetate under 760.051 Torr; Reflux; | 59% |
| With hydrogen In ethyl acetate at 50℃; under 1551.49 - 2585.81 Torr; for 20h; | 17% |
| With nickel In ethyl acetate Heating; |
-
-
110-00-9
furan

-
-
75532-25-1
2,2,4,4-tetrahydrothiophene-3,4-dicarboxylic acid anhydride

-
A
-
80558-50-5
epi-catharidin

-
B
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| nickel 1.) 7000 bar, 24 h, 2.) ethyl acetate, reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |


-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide; isopropyl alcohol Behandeln des Reaktionsprodukts mit konz. Schwefelsaeure; |

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With ozone; ethyl acetate at -40℃; Erhitzen des Reaktionsprodukts mit Essigsaeure und wss. Wasserstoffperoxid; |

| Conditions | Yield |
|---|---|
| at 150℃; im Rohr; |

| Conditions | Yield |
|---|---|
| at -40℃; Erhitzen des Reaktionsprodukts mit Essigsaeure und wss. Wasserstoffperoxid; |


-
-
56-25-7
cantharidin
-
-
7647-01-0
hydrogenchloride

-
-
853200-58-5
2-hydroxy-3a,7a-dimethyl-hexahydro-4,7-epoxido-isoindole-1,3-dione

-
A
-
7803-49-8
hydroxylamine

-
B
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| at 150℃; im Rohr; |


| Conditions | Yield |
|---|---|
| at 150 - 155℃; |

-
-
75532-26-2
C10H8O4S

-
A
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / 10percent Pd-C 2: Raney Ni / ethyl acetate / Heating View Scheme |
-
-
75532-26-2
C10H8O4S

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With hydrogen In water; ethyl acetate for 4h; Reflux; | 50 mg |
| Multi-step reaction with 2 steps 1: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 2: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
155251-30-2
3-cyano-3-hydroxy-tetrahydrothiophene-4-carboxylic acid methyl ester

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine; trichlorophosphate / benzene / 5 h / 20 - 52 °C 2.1: hydrogenchloride; water; acetic acid / Reflux 3.1: thionyl chloride / Reflux 4.1: 1-butyl-3-methylimidazolium Tetrafluoroborate / 19 h / 50 °C 4.2: Raney-Ni / 4 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydrogensulfite / water; methanol / 16 h / 20 °C / pH 7 - 8 2.1: pyridine; trichlorophosphate / benzene / 5 h / 20 - 52 °C 3.1: hydrogenchloride; water; acetic acid / Reflux 4.1: thionyl chloride / Reflux 5.1: 1-butyl-3-methylimidazolium Tetrafluoroborate / 19 h / 50 °C 5.2: Raney-Ni / 4 h / Reflux View Scheme | |
| Multi-step reaction with 7 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / 0.5 h / -30 - -20 °C / Inert atmosphere 2: tris-(dibenzylideneacetone)dipalladium(0); 1,1'-bis-(diphenylphosphino)ferrocene / N,N-dimethyl-formamide / 24 h / 50 °C / 2068.65 Torr 3: sodium hydroxide; water / tetrahydrofuran / 2.5 h 4: acetyl chloride / toluene / 4 h / Reflux 5: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 6: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 7: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
96307-21-0
3-cyano-2,5-dihydrothiophene-4-carboxylic acid methyl ester

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogenchloride; water; acetic acid / Reflux 2.1: thionyl chloride / Reflux 3.1: 1-butyl-3-methylimidazolium Tetrafluoroborate / 19 h / 50 °C 3.2: Raney-Ni / 4 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: methanol; lithium / 7 h / 20 °C / Inert atmosphere; Reflux 2: hydrogenchloride / water / pH Ca. 5 3: N-ethyl-N,N-diisopropylamine / dichloromethane / 0.5 h / -30 - -20 °C / Inert atmosphere 4: tris-(dibenzylideneacetone)dipalladium(0); 1,1'-bis-(diphenylphosphino)ferrocene / N,N-dimethyl-formamide / 24 h / 50 °C / 2068.65 Torr 5: sodium hydroxide; water / tetrahydrofuran / 2.5 h 6: acetyl chloride / toluene / 4 h / Reflux 7: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 8: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 9: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: hydrogenchloride / water / pH Ca. 5 2: N-ethyl-N,N-diisopropylamine / dichloromethane / 0.5 h / -30 - -20 °C / Inert atmosphere 3: tris-(dibenzylideneacetone)dipalladium(0); 1,1'-bis-(diphenylphosphino)ferrocene / N,N-dimethyl-formamide / 24 h / 50 °C / 2068.65 Torr 4: sodium hydroxide; water / tetrahydrofuran / 2.5 h 5: acetyl chloride / toluene / 4 h / Reflux 6: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 7: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 8: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
170945-21-8
methyl 4-{[(trifluoromethyl)sulfonyl]oxy}-2,5-dihydrothiophene-3-carboxylate

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: tris-(dibenzylideneacetone)dipalladium(0); 1,1'-bis-(diphenylphosphino)ferrocene / N,N-dimethyl-formamide / 24 h / 50 °C / 2068.65 Torr 2: sodium hydroxide; water / tetrahydrofuran / 2.5 h 3: acetyl chloride / toluene / 4 h / Reflux 4: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 5: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 6: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
20946-32-1
3,4-dimethyl 2,5-dihydrothiophene-3,4-dicarboxylate

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sodium hydroxide; water / tetrahydrofuran / 2.5 h 2: acetyl chloride / toluene / 4 h / Reflux 3: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 4: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 5: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
20688-07-7
2,2,4,4-tetrahydrothiophene-3,4-dicarboxylic acid

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: acetyl chloride / toluene / 4 h / Reflux 2: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 3: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 4: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |
-
-
75532-25-1
2,2,4,4-tetrahydrothiophene-3,4-dicarboxylic acid anhydride

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1-methyl-pyrrolidin-2-one / 48 h / 45 °C 2: hydrogen; 20 mol% Pd/C / ethyl acetate / 760.05 Torr 3: hydrogen / ethyl acetate / 760.05 Torr / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| In water 1.) reflux, 4 h, 2.) 200 deg C, 1 h; | 93% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 92% |
-
-
13650-49-2
Nδ-(tert-butoxycarbonyl)-L-ornithine

-
-
56-25-7
cantharidin

-
-
849355-19-7
(S)-5-tert-Butoxycarbonylamino-2-((1S,2R,6S,7R)-2,6-dimethyl-3,5-dioxo-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-4-yl)-pentanoic acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 48h; | 92% |
-
-
56-25-7
cantharidin

-
-
76970-77-9
7-oxabicyclo<2.2.1>heptane-2,3-dimethyl-2,3-dicarboxylic acid imide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 180℃; for 2h; | 91% |
| With ammonium hydroxide 1.) reflux, 2 h, 2.) 200 deg C, 1 h; | 66% |
| (i) aq. NH3, (ii) (heating); Multistep reaction; | |
| With ammonia |
-
-
17467-15-1
2-amino-4-phenyl-1,3,5-thiadiazole

-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 91% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 89% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 180℃; for 24h; | 89% |

| Conditions | Yield |
|---|---|
| With acetic acid Sealed tube; | 89% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 88% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 86% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 180℃; for 24h; | 86% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 180℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 84% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 2.5h; Acylation; Heating; | 80.1% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 79% |
| With triethylamine In toluene at 200℃; for 2h; | 52% |

| Conditions | Yield |
|---|---|
| With acetic acid at 135℃; for 12h; Sealed tube; | 79% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 77% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 76% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 74% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 180℃; for 5h; | 73% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 72% |
| With triethylamine In toluene at 200℃; for 2h; | 62% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 69% |
-
-
56-25-7
cantharidin

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 200℃; for 2h; | 69% |
Cantharidin Chemical Properties
Product Name: Cantharidin
Synonyms: (2R,6S)-2,6-Dimethyl-4,10-dioxatricyclo[5.2.1.0~2,6~]decan-3,5-dion ; (2R,6S)-2,6-Dimethyl-4,10-dioxatricyclo[5.2.1.0~2,6~]decane-3,5-dione ; (2R,6S)-2,6-Diméthyl-4,10-dioxatricyclo[5.2.1.0~2,6~]décane-3,5-dione ; (3aR,7aS)-3a,7a-Dimethylhexahydro-4,7-epoxy-2-benzofuran-1,3-dione
Molecular Structure of Cantharidin (CAS NO.56-25-7) : 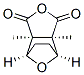
Molecular Formula of Cantharidin (CAS NO.56-25-7) : C10H12O4
Molecular Weight of Cantharidin (CAS NO.56-25-7) : 196.20
CAS NO: 56-25-7
EINECS: 200-263-3
Product Categories: chiral
Mol File: 56-25-7.mol
Merck : 13,1757
Index of Refraction: 1.547
Surface Tension: 49.2 dyne/cm
Density: 1.362 g/cm3
Flash Point: 146.2 °C
Enthalpy of Vaporization: 56.91 kJ/mol
Boiling Point: 326.9 °C at 760 mmHg
Vapour Pressure: 0.00021 mmHg at 25°C
Melting point 215-217 ºC
Appearance:white to light yellow crystal powder
Cantharidin History
Cantharidin was first isolated by Pierre Robiquet in 1810. It is an odorless and colorless solid at room temperature. It is secreted by the male blister beetle and given to the female during the mating. Afterwards the female beetle will cover its eggs with it as a defense against predators. The complete mechanism of the biosynthesis is currently unknown. If cantharidin is ingested, it severely irritates the urinary tract as it is excreted, causing swelling of the genitalia. This can cause a harmful condition known as priapism in men, where an erection lasts more than about four hours.
Cantharidin Uses
1. Cantharidin (CAS NO.56-25-7) is used in biochemical research, clinically as antineoplastic agents, mainly for primary liver cancer, It is often used in conjunction with other antineoplastic agents.
2. Cantharidin (CAS NO.56-25-7) is used as hydroxycanthayridimide intermediates.
Cantharidin Production
Raw materials Magnesium stearate -->MAGNESIUM TRISILICATE-->Oils, animal, mixed with vegetable oil Me esters, sulfurized -->Pioneer Blue Natural Rubber Latex Gloves, Size 9
Cantharidin (CAS NO.56-25-7) can be extracted from the insect cantharis body,also can be chemically synthesized.
Cantharidin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | oral | 310mg/kg (310mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| chicken | LDLo | subcutaneous | 75mg/kg (75mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| dog | LDLo | oral | 50mg/kg (50mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| frog | LDLo | oral | 7273mg/kg (7273mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| human | LDLo | oral | 428ug/kg (0.428mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 646, 1969. | |
| mammal (species unspecified) | LD50 | intraperitoneal | 1710ug/kg (1.71mg/kg) | "Zhongliu Yanjiu" Cancer Review, Yu, R., et al., eds., Shanghai Science/Technology Publisher,Peop. Rep. China, 1994Vol. -, Pg. 183, 1994. | |
| mammal (species unspecified) | LDLo | oral | 75mg/kg (75mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| mammal (species unspecified) | LDLo | unreported | 50mg/kg (50mg/kg) | Zhongcaoyao. Chinese Traditional and Herbal Medicine. Vol. 20, Pg. 135, 1989. | |
| mouse | LD50 | intraperitoneal | 1mg/kg (1mg/kg) | Journal of Agricultural and Food Chemistry. Vol. 35, Pg. 823, 1987. | |
| pigeon | LDLo | oral | 32mg/kg (32mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| rabbit | LDLo | oral | 20mg/kg (20mg/kg) | CARDIAC: ARRHYTHMIAS (INCLUDING CHANGES IN CONDUCTION) | Toxicon. Vol. 23, Pg. 36, 1985. |
| rabbit | LDLo | subcutaneous | 1mg/kg (1mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. |
Cantharidin Safety Profile
Hazard Codes  T+T
T+T
Risk Statements 28-36/37/38-23/24/25
R28:Very toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
Safety Statements 53-45-36/37/39
S53:Avoid exposure - obtain special instructions before use.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
RIDADR UN 2811 6.1/PG 1
WGK Germany 3
RTECS RN8575000
HazardClass 6.1(a)
PackingGroup I
Cantharidin Specification
1.General Description: Brown to black powder or plates or scales. Formerly used as a counterirritant and vesicant. Used for the removal of warts. Used as an experimental anti tumor agent. Active ingredient in spanish fly, a reputed aphrodisiac.
2.Reactivity Profile: Organic anhydrides, such as Cantharidin, are incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
3.Health Hazard :Cantharidin is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste of less than 7 drops for a 70 kg (150 lb.) person. It is very toxic by absorption through skin.
4.Fire Hazard: When heated to decomposition Cantharidin emits acrid smoke and irritating fumes.
Related Products
- Cantharidin
- 5625-78-5
- 5625-90-1
- 56259-20-2
- 5625-98-9
- 56261-48-4
- 56265-06-6
- 56265-09-9
- 56265-46-4
- 56267-47-1
- 56267-48-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View