-
Name
Carvedilol
- EINECS 1308068-626-2
- CAS No. 72956-09-3
- Article Data46
- CAS DataBase
- Density 1.25 g/cm3
- Solubility 449.2ug/L(22.5 oC)
- Melting Point 113-117 °C
- Formula C24H26N2O4
- Boiling Point 655.2 °C at 760 mmHg
- Molecular Weight 496.517
- Flash Point 350.1 °C
- Transport Information UN 3077 9/PG 3
- Appearance colourless crystalline powder
- Safety 61-36-26
- Risk Codes 51/53-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  N
N
- Synonyms 2-Propanol, 1-(9H-carbazol-4-yloxy)-3-((2-(2-methoxyphenoxy)ethyl)amino)-, (+-)-;Carvedilolum [Latin];Carvedilol [USAN:BAN:INN:JAN];Coreg (TN);Carvedilol (JAN/USAN);2-Propanol,1-(9H-carbazol-4-yloxy)-3-[[2- (2-methoxyphenoxy)ethyl]amino]-;SKF 105517;(+-)-1-(Carbazol-4-yloxy)-3-((2-(o-methoxyphenoxy)ethyl)amino)-2-propanol;DQ 2466;1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethylamino]propan-2-ol;
- PSA 75.74000
- LogP 4.12890
Synthetic route
-
-
787598-91-8
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate

-
A
-
918903-20-5
C39H39N3O6

-
B
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; isopropyl alcohol at 50 - 70℃; for 0.75h; | A n/a B 91.21% |
| Stage #1: (+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate With triethylamine In ethyl acetate at 10 - 35℃; for 1h; Stage #2: With sodium chloride In water; ethyl acetate for 0.5h; |
-
-
180987-80-8
5-(((9H-carbazol-4-yl)oxy)methyl)-3-(2-(2-methoxyphenoxy)ethyl)oxazolidin-2-one

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In propan-1-ol at 60℃; for 4h; Solvent; Temperature; | 89.11% |
| With sodium hydroxide In ethanol Reflux; Inert atmosphere; | 70% |
| With ethanol; sodium hydroxide Inert atmosphere; Reflux; | 70% |
-
-
1016214-10-0
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol tolsylate

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; ethyl acetate Product distribution / selectivity; | 88% |
| With sodium carbonate In water; ethyl acetate | 80% |
| With sodium hydroxide; water In isopropyl alcohol Product distribution / selectivity; |
-
-
72955-94-3
1-[benzyl[2(2-methoxyphenoxy)ethyl]amino]-3-(9H-carbazol-4-yloxy)propan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In water; ethyl acetate at 20 - 50℃; for 8h; | 84% |
| With hydrogen; palladium 10% on activated carbon In methanol at 70℃; under 2942.29 - 4413.43 Torr; | 76.7% |
| With hydrogen; 5%-palladium/activated carbon In ethyl acetate at 60 - 70℃; under 2574.5 - 3310.08 Torr; for 10h; |
-
-
143412-40-2, 143412-41-3, 72955-96-5
1-amino-3-(9H-carbazol-4-yloxy)-propan-2-ol

-
-
53815-60-4
1-(2-chloroethoxy)-2-methoxybenzene

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 80 - 85℃; | 83% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Inert atmosphere; Reflux; | 81% |
| With N-ethyl-N,N-diisopropylamine In 1,2-dimethoxyethane at 80 - 85℃; | 36.26% |
| With sulfuric acid; potassium carbonate In isopropyl alcohol at 80℃; for 6h; |

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 30 - 40℃; for 17h; | 67% |
-
-
72956-09-3
(+/-) 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethyl]amino-2-propanol oxalate

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With ammonia In dichloromethane; water at 20℃; for 1h; pH=9.0 - 9.3; | 63.2% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
A
-
918903-20-5
C39H39N3O6

-
B
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 68 - 72℃; for 18 - 20h; | A n/a B 58% |

-
-
64464-07-9
2-[(2-methoxy)phenoxy]ethylamine hydrochloride

-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Stage #1: 2-[(2-methoxy)phenoxy]ethylamine hydrochloride With sodium hydroxide In water at 20℃; pH=9 - 9.5; Stage #2: 4-(2,3-epoxypropoxy)carbazole In water at 80 - 85℃; for 0.75 - 1h; Product distribution / selectivity; | 45.99% |
| Stage #1: 2-[(2-methoxy)phenoxy]ethylamine hydrochloride With sodium hydroxide In water; isopropyl alcohol at 20℃; pH=9 - 9.5; Stage #2: 4-(2,3-epoxypropoxy)carbazole In water; isopropyl alcohol for 4 - 5h; Product distribution / selectivity; Heating / reflux; | 43.05% |
| With potassium carbonate In i-Amyl alcohol at 80 - 85℃; for 7h; | 41% |
| With calcium carbonate In isopropyl alcohol at 80℃; for 4h; |
-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Stage #1: 2-[(2-methoxy)phenoxy]ethylamine hydrochloride With potassium carbonate In isopropyl alcohol at 35℃; for 0.25h; Stage #2: 4-(2,3-epoxypropoxy)carbazole In isopropyl alcohol at 83℃; for 5h; | 45% |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 4 h / 140 °C 2: methylamine / water / 2 h / 80 °C 3: potassium carbonate / toluene / 80 - 85 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrogenchloride / water; methanol / 0 - 30 °C 2: potassium carbonate / N,N-dimethyl-formamide / 2 h / 90 - 95 °C 3: methylamine / water / 2 h / 80 °C 4: dipotassium hydrogenphosphate; N-benzyl-N,N,N-triethylammonium chloride / toluene / 5 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: 4-methoxypyridine N-oxide; sodium sulfate / dichloromethane / 60 h / 20 °C 2: potassium carbonate / toluene / 60 h / Reflux 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 17 h / 30 - 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: water; hydrogen bromide / isopropyl alcohol / 25 - 60 °C 2: triethylamine / isopropyl alcohol / 60 °C View Scheme |
-
-
787598-91-8
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol Product distribution / selectivity; |
-
-
374779-43-8
(±)-1-(9H-carbazol-4-yloxy)-3-{[2-(2-methoxyphenoxy)ethyl]amino}propan-2-ol sulfate

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; ethyl acetate Product distribution / selectivity; |
-
-
1187921-88-5
C27H34N2O4Si

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Stage #1: C27H34N2O4Si With phosphoric acid; water In ethyl acetate at 20 - 25℃; Stage #2: With ammonia In water; ethyl acetate pH=~ 9.5; Product distribution / selectivity; |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1187921-94-3
1-(9H-carbazol-4-yloxy)-3-bromopropan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol at 60℃; Product distribution / selectivity; |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1187921-93-2
1-(9H-carbazol-4-yloxy)-3-chloropropan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 80 - 85℃; for 13h; Product distribution / selectivity; |
-
-
52602-39-8
9H-carbazol-4-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / water; dimethyl sulfoxide / 12 h / 25 - 45 °C 2: 1,2-dimethoxyethane; ethyl acetate View Scheme | |
| Multi-step reaction with 5 steps 1.1: pyridine / isopropyl alcohol / 0.17 h / Inert atmosphere 1.2: 16 h / 15 - 20 °C / Inert atmosphere 2.1: pyridine / dichloromethane / 12 h / 5 - 20 °C / Inert atmosphere 3.1: dmap / N,N-dimethyl-formamide / 15 - 30 °C / Inert atmosphere 4.1: 100 - 110 °C / Inert atmosphere 5.1: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: pyridine / isopropyl alcohol / 0.17 h / Inert atmosphere 1.2: 16 h / 15 - 20 °C / Inert atmosphere 2.1: dichloromethane / 4 h / 0 °C / Inert atmosphere 3.1: potassium carbonate / N,N-dimethyl-formamide / 10 h / 100 °C / Inert atmosphere 4.1: 100 - 110 °C / Inert atmosphere 5.1: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme |
-
-
1187921-93-2
1-(9H-carbazol-4-yloxy)-3-chloropropan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine / dichloromethane / 12 h / 5 - 20 °C / Inert atmosphere 2: dmap / N,N-dimethyl-formamide / 15 - 30 °C / Inert atmosphere 3: 100 - 110 °C / Inert atmosphere 4: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: dichloromethane / 4 h / 0 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 10 h / 100 °C / Inert atmosphere 3: 100 - 110 °C / Inert atmosphere 4: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 2 h / 90 - 95 °C 2: methylamine / water / 2 h / 80 °C 3: dipotassium hydrogenphosphate; N-benzyl-N,N,N-triethylammonium chloride / toluene / 5 h / Reflux View Scheme |
-
-
1392322-33-6
3-((9H-carbazol-4-yloxy)methyl)-3-chloropropane-2-yl phenyl carbonate

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dmap / N,N-dimethyl-formamide / 15 - 30 °C / Inert atmosphere 2: 100 - 110 °C / Inert atmosphere 3: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme |

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 10 h / 100 °C / Inert atmosphere 2: 100 - 110 °C / Inert atmosphere 3: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dmap / N,N-dimethyl-formamide / 15 - 30 °C / Inert atmosphere 2: 100 - 110 °C / Inert atmosphere 3: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 10 h / 100 °C / Inert atmosphere 2: 100 - 110 °C / Inert atmosphere 3: ethanol; sodium hydroxide / Inert atmosphere; Reflux View Scheme |
-
-
1218777-33-3
5-((9H-carbazol-4-yloxy)methyl)-oxazolidin-2-one

-
-
53815-60-4
1-(2-chloroethoxy)-2-methoxybenzene

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 25 h / 25 - 100 °C / Inert atmosphere 2: sodium hydroxide / ethanol / 12 h / Reflux; Inert atmosphere View Scheme |
-
-
1392100-68-3
1-(9H-carbazol-4-yloxy)-3-(benzhydrylamino)propan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: palladium 10% on activated carbon / methanol / 6 h / 3102.97 - 3620.13 Torr / Inert atmosphere 2: 0 °C / Reflux; basic condition; Inert atmosphere 3: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 25 h / 25 - 100 °C / Inert atmosphere 4: sodium hydroxide / ethanol / 12 h / Reflux; Inert atmosphere View Scheme |
-
-
143412-40-2, 143412-41-3, 72955-96-5
1-amino-3-(9H-carbazol-4-yloxy)-propan-2-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0 °C / Reflux; basic condition; Inert atmosphere 2: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 25 h / 25 - 100 °C / Inert atmosphere 3: sodium hydroxide / ethanol / 12 h / Reflux; Inert atmosphere View Scheme |
-
-
1218777-33-3
5-((9H-carbazol-4-yloxy)methyl)-oxazolidin-2-one

-
-
1187921-84-1
1-(2-iodoethoxy)-2-methoxybenzene

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 36 h / 100 °C 2: water; sodium hydroxide / ethanol / 12 h / Reflux View Scheme |
-
-
90-05-1
2-methoxy-phenol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: tetrabutylammomium bromide; sodium hydroxide / water / 5 h / 80 °C 2: sodium iodide / acetone / 48 h / Reflux 3: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 36 h / 100 °C 4: water; sodium hydroxide / ethanol / 12 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide; tetrabutylammomium bromide / water / 0.25 h / 30 °C 1.2: 20 - 70 °C 2.1: sodium hydroxide / toluene / 0.17 h / 25 - 30 °C 2.2: 25 - 30 °C 3.1: dipotassium hydrogenphosphate; N-benzyl-N,N,N-triethylammonium chloride / toluene / 5 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: tetrabutylammomium bromide; sodium hydroxide / 16 h / Reflux 2: water; sodium hydroxide / 13 h / 130 °C 3: ethyl acetate / 24 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: tetrabutylammomium bromide; sodium hydroxide / 16 h / Reflux 2: tetrabutylammomium bromide / 180 - 185 °C 3: water; potassium hydroxide / 13 h / 130 °C 4: ethyl acetate / 24 h / Reflux View Scheme |
-
-
53815-60-4
1-(2-chloroethoxy)-2-methoxybenzene

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium iodide / acetone / 48 h / Reflux 2: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 36 h / 100 °C 3: water; sodium hydroxide / ethanol / 12 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: water; sodium hydroxide / 13 h / 130 °C 2: ethyl acetate / 24 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: tetrabutylammomium bromide / 180 - 185 °C 2: water; potassium hydroxide / 13 h / 130 °C 3: ethyl acetate / 24 h / Reflux View Scheme |
-
-
1207276-96-7
sodium salt of 9H-carbazol-4-ol

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 48 h / 100 °C 2: potassium carbonate; potassium iodide / N,N-dimethyl-formamide / 36 h / 100 °C 3: water; sodium hydroxide / ethanol / 12 h / Reflux View Scheme |
-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With deuterium In tetrahydrofuran under 1500.15 Torr; for 24h; Glovebox; Heating; | 99% |
-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With phosphoric acid In acetonitrile at 20℃; for 0.5h; Product distribution / selectivity; | 98% |
| With phosphoric acid In acetone Product distribution / selectivity; | 98% |
| With phosphoric acid In methanol Product distribution / selectivity; | 98% |
-
-
72956-09-3
carvedilol

-
-
374779-45-0
1-(9H-carbazole-4-oxy)-3-[2-(2-methoxyphenoxy)ethylamino]-2-propanolphosphate

| Conditions | Yield |
|---|---|
| With phosphoric acid In water; acetonitrile at 20 - 70℃; Product distribution / selectivity; | 97% |
| With phosphoric acid In water; N,N-dimethyl-formamide; isopropyl alcohol at 20℃; Product distribution / selectivity; | 96% |
| With phosphorus pentoxide In water; acetone at 0 - 45℃; Product distribution / selectivity; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 1h; | 96% |
-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide In water; acetone at 0 - 45℃; for 1h; Product distribution / selectivity; | 95% |
| With hydrogenchloride; dipotassium hydrogenphosphate In water; acetone at 0 - 45℃; for 1h; pH=4.5 - 5; Product distribution / selectivity; | 94.49% |
| With PPA In water; acetone at 0 - 45℃; for 1h; Product distribution / selectivity; | 93.3% |
-
-
72956-09-3
carvedilol

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
180987-80-8
5-(((9H-carbazol-4-yl)oxy)methyl)-3-(2-(2-methoxyphenoxy)ethyl)oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 5h; | 93% |
| With triethylamine In dichloromethane at 20℃; |
-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol; water | 92.1% |
| With sulfuric acid In water; acetone at 17 - 35℃; for 10 - 24h; |
-
-
72956-09-3
carvedilol

-
-
320401-51-2
2'-(((2S,3R,4S,5S)-3,4,5-triacetoxy-6-(methoxycarbonyl)tetrahydro-2H-pyran-2-yloxy)carbonylamino)biphenyl-2-carboxylic acid

-
A
-
1015-89-0
6(5H)-phenanthridinone

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane | A n/a B 92% |

-
-
72956-09-3
carvedilol

-
-
374779-43-8
(±)-1-(9H-carbazol-4-yloxy)-3-{[2-(2-methoxyphenoxy)ethyl]amino}propan-2-ol sulfate

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol; water | 89.6% |
| With sulfuric acid In dichloromethane; water pH=4.0 - 4.5; | |
| With sulfuric acid In water; acetone at 17 - 35℃; for 10 - 24h; |

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 95℃; for 18h; | 77% |
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 95℃; for 18h; Product distribution / selectivity; | 75% |
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 95℃; |

| Conditions | Yield |
|---|---|
| Stage #1: carvedilol With potassium tert-butylate In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: 4-cyano-N,N,N-trimethylanilinium trifluoromethansulfonate In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 3h; Inert atmosphere; Schlenk technique; | 71% |

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; alpha-D-glucopyranose; maltose binding protein-RebF; RebH variant 4-V; nicotinamide adenine dinucleotide; flavin adenine dinucleotide; sodium chloride In methanol at 25℃; for 72h; pH=7.4; Enzymatic reaction; | 69% |

| Conditions | Yield |
|---|---|
| With water In acetone at 20 - 40℃; Product distribution / selectivity; | 67% |
| With water In acetone at 20 - 40℃; for 24 - 48h; Product distribution / selectivity; | 67% |
| With water | |
| With water Product distribution / selectivity; |
-
-
72956-09-3
carvedilol

-
-
105-58-8
Diethyl carbonate

-
-
180987-80-8
5-(((9H-carbazol-4-yl)oxy)methyl)-3-(2-(2-methoxyphenoxy)ethyl)oxazolidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: carvedilol In methanol Stage #2: With methanol; sodium In methanol Stage #3: Diethyl carbonate In methanol for 12h; Product distribution / selectivity; Heating / reflux; | 65% |
-
-
72956-09-3
carvedilol

-
-
3582-05-6
trifluoromethyl trifluoromethanesulfonate

-
-
180987-80-8
5-(((9H-carbazol-4-yl)oxy)methyl)-3-(2-(2-methoxyphenoxy)ethyl)oxazolidin-2-one

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 1h; Sealed tube; | 61% |
-
-
72956-09-3
carvedilol

-
-
611-71-2, 611-72-3, 17199-29-0, 90-64-2
MANDELIC ACID

-
-
852995-78-9
carvedilol mandelate

| Conditions | Yield |
|---|---|
| In methanol; water; acetone at 17 - 35℃; for 10 - 24h; | 54.5% |
| In methanol; water; acetone at 17 - 35℃; for 10 - 24h; | 54.5% |
-
-
72956-09-3
carvedilol

-
-
74-88-4
methyl iodide

-
-
72956-35-5
1-(9H-carbazol-4-yloxy)-3-{[2-(2-methoxyphenoxy)ethyl]methylamino}-2-propanol

| Conditions | Yield |
|---|---|
| Stage #1: carvedilol With sodium hydride In tetrahydrofuran at 0℃; for 0.333333h; Stage #2: methyl iodide In tetrahydrofuran at 0 - 20℃; for 4h; | 54% |
| With sodium hydride In tetrahydrofuran at 20℃; for 4h; | 54% |
| In tetrahydrofuran at 20℃; |

| Conditions | Yield |
|---|---|
| Stage #1: carvedilol With sodium hydride In tetrahydrofuran at 0℃; for 0.333333h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 4h; | 49% |
Carvedilol Chemical Properties
Structure of Carvedilol (CAS NO.72956-09-3):
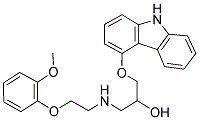
IUPAC Name: 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethylamino]propan-2-ol
Empirical Formula: C24H26N2O4
Molecular Weight: 406.4742g/mol
Index of Refraction: 1.657
Molar Refractivity: 119.59 cm3
Molar Volume: 325.1 cm3
Polarizability: 47.41×10-24cm3
Surface Tension: 53.9 dyne/cm
Density: 1.25 g/cm3
Flash Point: 350.1 °C
Enthalpy of Vaporization: 101.39 kJ/mol
Melting Point: 113-117°C
Boiling Point: 655.2 °C at 760 mmHg
Vapour Pressure: 4.63E-18 mmHg at 25°C
Product Categories: Active Pharmaceutical Ingredients;Intermediates & Fine Chemicals;Pharmaceuticals;API's;Adrenoceptor
Canonical SMILES: COC1=CC=CC=C1OCCNCC(COC2=CC=CC3=C2C4=CC=CC=C4N3)O
InChI: InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3
InChIKey: OGHNVEJMJSYVRP-UHFFFAOYSA-N
Carvedilol Uses
Carvedilol (CAS NO.72956-09-3) is a beta blocker and an alpha blocker indicated in the treatment of mild to moderate congestive heart failure (CHF).
Carvedilol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 1gm/kg (1000mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 20, Pg. 1181, 1989. |
| mouse | LD50 | intraperitoneal | 364mg/kg (364mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | New Cardiovascular Drugs. Vol. 5, Pg. 135, 1987. |
| mouse | LD50 | intravenous | 27mg/kg (27mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LIVER: OTHER CHANGES | New Cardiovascular Drugs. Vol. 5, Pg. 135, 1987. |
| mouse | LDLo | oral | 8gm/kg (8000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 20, Pg. 1181, 1989. |
| rat | LD50 | intraperitoneal | 769mg/kg (769mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | New Cardiovascular Drugs. Vol. 5, Pg. 135, 1987. |
| rat | LD50 | intravenous | 25mg/kg (25mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LIVER: OTHER CHANGES | New Cardiovascular Drugs. Vol. 5, Pg. 135, 1987. |
| rat | LDLo | oral | 8gm/kg (8000mg/kg) | GASTROINTESTINAL: ULCERATION OR BLEEDING FROM STOMACH BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 20, Pg. 1181, 1989. |
Carvedilol Safety Profile
Hazard Codes of Carvedilol (CAS NO.72956-09-3):  N,
N, Xn
Xn
Risk Statements: 51/53-36/37/38-20/21/22
R51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R36/37/38: Irritating to eyes, respiratory system and skin.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 61-36-26
S61: Avoid release to the environment. Refer to special instructions / safety data sheets.
S36: Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR: UN 3077 9/PG 3
RTECS: UA8670000
Carvedilol Specification
Carvedilol , its cas register number is 72956-09-3. It also can be called (+-)-1-(Carbazol-4-yloxy)-3-((2-(o-
methoxyphenoxy)ethyl)amino)-2-propanol ; (+-)-1-Carbazol-4-yloxy)-3-((2-(o-methoxyphenoxy)ethyl)amino)-2-propanol ;
2-Propanol, 1-(9H-carbazol-4-yloxy)-3-((2-(2-methoxyphenoxy)ethyl)amino)-, (+-)- ; BM 14190 ; Carvedilolum ; Coreg ;
DQ 2466 ; HSDB 7044 ; SKF 105517 ; UNII-0K47UL67F2 . Carvedilol (CAS NO.72956-09-3) is a colourless crystalline solid. Side effects of carvedilol were observed to include nightmares,sleep disturbances and anxiety and panic attacks. This is based on a single case report of a patient already diagnosed with anxiety disorder and using the drug improperly before bedtime at elevated doses.
Related Products
- Carvedilol
- Carvedilol phosphate hemihydrate
- 72956-44-6
- 729572-33-2
- 72957-37-0
- 72957-38-1
- 7295-76-3
- 7295-85-4
- 729607-74-3
- 7296-20-0
- 72962-43-7
- 72963-72-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View