-
Name
Cyclohexanol
- EINECS 203-630-6
- CAS No. 108-93-0
- Article Data1857
- CAS DataBase
- Density 0.968 g/cm3
- Solubility 3.6 g/100 mL (20 °C)
- Melting Point 23 °C
- Formula C6H12O
- Boiling Point 159.552 °C at 760 mmHg
- Molecular Weight 100.161
- Flash Point 67.778 °C
- Transport Information UN 1993
- Appearance a colorless liquid with a camphor-like odor
- Safety 24/25
- Risk Codes 20/22-37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1-Cyclohexanol;Adronal;Adronol;Anol;Cyclohexyl alcohol;Hexahydrophenol;Hexalin;Hexalin(alcohol);Hydroxycyclohexane;NSC 403656;NSC 54711;Naxol;Phenol, hexahydro-;
- PSA 20.23000
- LogP 1.31140
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In decane at 349.84℃; under 22502.3 Torr; for 3h; Temperature; Autoclave; | 95.6% |
| With hydrogen In water at 160℃; for 6h; | 80% |
| Multi-step reaction with 3 steps 1: hydrogenchloride / 80 °C / 760.05 Torr / Electrochemical reaction 2: sodium hydroxide / 80 °C / Electrochemical reaction 3: hydrogenchloride / 80 °C / 760.05 Torr / Electrochemical reaction View Scheme |
-
-
156147-58-9
1-((cyclohexyloxy)methyl)-4-methoxybenzene

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With 4,4'-bipyridine; oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 80℃; under 3000.3 Torr; for 10h; Reagent/catalyst; Autoclave; Green chemistry; | 96% |
| With 4,4'-bipyridine; (phthalocyaninato)iron(II); oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 80℃; under 3000.3 Torr; for 10h; Autoclave; | 91% |
| With tert.-butylnitrite; oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In monoethylene glycol diethyl ether at 120℃; under 1500.15 Torr; for 1.5h; | |
| With tert.-butylnitrite; oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In chlorobenzene at 100℃; under 760.051 Torr; for 3.5h; |

| Conditions | Yield |
|---|---|
| With water; hydrogen In tert-butyl alcohol at 180℃; under 33003.3 Torr; for 8h; Reagent/catalyst; Pressure; Temperature; Molecular sieve; Autoclave; | 99.9% |
| With water; hydrogen at 160℃; Hydrogenation.Leiten ueber Nickel/Bimsstein; |

| Conditions | Yield |
|---|---|
| With hydrogen; tetra(n-butyl)ammonium hydrogensulfate; rhodium colloidal catalyst In water at 36℃; under 180018 Torr; for 62h; pH=7.5; Catalytic hydrogenation; | 100% |
| In methanol; water | 100% |
| With hydrogen In water at 70℃; under 7500.75 Torr; for 0.25h; | 100% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; water; triethylamine In acetonitrile at 20℃; for 0.166667h; | 92% |
| With Fe2O3-SiO2 nanoparticles; air In water at 50℃; for 3h; Green chemistry; | 91% |
| With dihydrogen peroxide at 30℃; for 5h; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With hydrogen; 5 percent Rh/MgO; magnesium oxide In water at 323℃; under 15001.2 Torr; Product distribution; | 100% |
| With Triethoxysilane; benzoic acid ethyl ester; cesium fluoride at 25℃; for 0.0166667h; | 100% |
| With zinc(II) tetrahydroborate In acetonitrile for 0.166667h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With Geotrichum candidum CCT 1205 whole cells In aq. phosphate buffer at 28℃; for 24h; pH=6.5; Enzymatic reaction; | A 100% B n/a |
| With hydrogen; In methanol at 30℃; under 735.5 Torr; for 5h; | A 0.8% B 98.2% |
| With C8H13O2S2(3-)*Na(1+)*Rh(1+)*H(1+); hydrogen In water; toluene at 60℃; under 15001.5 Torr; for 4h; Inert atmosphere; Schlenk technique; Autoclave; | A 90% B 9% |

| Conditions | Yield |
|---|---|
| With ferredoxin reductase; [2Fe–2S] ferredoxin; cytochrome P450 enzyme CYP101B1 In aq. buffer pH=7.4; Kinetics; Reagent/catalyst; Enzymatic reaction; | 95% |
| With C44H34N8O9Ti; dihydrogen peroxide; sodium hydrogencarbonate In acetonitrile at 80℃; under 760.051 Torr; for 4h; Catalytic behavior; Reagent/catalyst; | 93% |
| With perchloric acid; dihydrogen peroxide In water; acetonitrile; tert-butyl alcohol at 60℃; for 1h; stereoselective reaction; | 92% |
-
-
90-05-1
2-methoxy-phenol

-
A
-
110-82-7
cyclohexane

-
B
-
2979-24-0
2-methoxycyclohexanol

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With 5% active carbon-supported ruthenium; hydrogen; magnesium oxide In water at 159.84℃; under 11251.1 Torr; for 2h; Reagent/catalyst; Autoclave; | A 7% B 12% C 79% |
| With Ni-doped silica; hydrogen In decalin at 140℃; under 22502.3 Torr; for 5h; Autoclave; | A 16.8% B 67.4% C 8.9% |
| With hydrogen In decane at 20 - 250℃; | A 25 %Chromat. B 9 %Chromat. C 8 %Chromat. |

-
-
101-84-8
diphenylether

-
A
-
110-82-7
cyclohexane

-
B
-
4645-15-2
dicyclohexyl ether

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In methyl cyclohexane at 90℃; under 37503.8 Torr; for 2.5h; chemoselective reaction; | |
| With hydrogen In decalin at 120℃; under 15001.5 Torr; for 2h; | |
| With hydrogen In methyl cyclohexane at 200℃; under 37503.8 Torr; for 0.666667h; Glovebox; Inert atmosphere; Autoclave; | |
| With carbon dioxide; rhodium on carbon; hydrogen In tetrahydrofuran; water at 80℃; for 5h; | |
| With hydrogen In water at 250℃; under 30003 Torr; for 2h; |


| Conditions | Yield |
|---|---|
| With hydrogen In Hexadecane at 260℃; under 7600.51 Torr; for 5h; Autoclave; | A 8.3% B 9.5% C 53.8% |


| Conditions | Yield |
|---|---|
| With phosphoric acid; 5 wt% ruthenium/carbon; hydrogen In water at 199.84℃; under 15001.5 Torr; for 2h; Autoclave; | |
| With hydrogen In dodecane at 219.84℃; under 15001.5 Torr; Reagent/catalyst; Autoclave; Inert atmosphere; | |
| With hydrogen In water at 200℃; under 7500.75 Torr; for 4h; | |
| With hydrogen at 299.84℃; |


| Conditions | Yield |
|---|---|
| With water; titanium tetrachloride; zinc In diethyl ether at 40℃; for 15h; Solvent; Inert atmosphere; | A 78.5% B 21.5% |
| With samarium; N-Bromosuccinimide In methanol at 20℃; for 0.5h; | A 70% B 10% |
| With chloro-trimethyl-silane; samarium diiodide; tert-butylammonium hexafluorophosphate(V) In tetrahydrofuran Electrochemical reaction; | A 59% B 40% |
| With methanol; samarium; iodine; allyl bromide In tetrahydrofuran at 20℃; Inert atmosphere; | A 40% B 36% |
| With diethyl ether; sodium |

| Conditions | Yield |
|---|---|
| With hydrogen at 160 - 200℃; under 1125.11 Torr; for 1h; Reagent/catalyst; Autoclave; | A 94.2% B 5.6% |
| With water In methanol at 220℃; under 22502.3 Torr; for 0.5h; Inert atmosphere; Microwave irradiation; | A 87.34% B 5.73% |
| With hydrogen; 1-butyl-3-methylimidazolium Tetrafluoroborate; Rh nanoparticles stabilized by poly(NVP-co-VBIMCl) at 75℃; under 30402 Torr; for 12h; Product distribution; | A 29% B 71% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen; magnesium oxide; ruthenium In water at 99.9℃; under 15001.2 Torr; Product distribution; | A 6.5% B 93.5% |
| With ammonia; hydrogen In methanol; ethanol at 100℃; under 37503.8 Torr; for 3h; Catalytic behavior; Reagent/catalyst; Autoclave; | A 93.4% B 6.6% |
| With ammonia; hydrogen In methanol at 80℃; for 6h; Autoclave; | A 91% B 9% |

| Conditions | Yield |
|---|---|
| With platinum on activated charcoal; water; aluminium at 20 - 80℃; for 36h; Sealed tube; | A 17% B 67 %Chromat. |
| With hydrogen; Rh on carbon In methanol at 20℃; under 760.051 Torr; for 2.5h; | |
| With carbon dioxide; rhodium on carbon; hydrogen In tetrahydrofuran; water at 80℃; for 5h; Solvent; | |
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; C23H36N(1+)*BF4(1-); potassium tert-butylate; hydrogen In tetrahydrofuran at 25℃; under 5168.35 Torr; for 2h; chemoselective reaction; | A 70 %Chromat. B 30 %Chromat. |
| With hydrogen In water at 80℃; under 11251.1 Torr; for 24h; | A 82 %Chromat. B 18 %Chromat. |

| Conditions | Yield |
|---|---|
| With water In aq. phosphate buffer at 25℃; for 7.5h; pH=2.7 - 3.2; Electrochemical reaction; | A 77% B 22% |

| Conditions | Yield |
|---|---|
| With water In aq. phosphate buffer at 25℃; for 1.5h; pH=2.7 - 3.2; Electrochemical reaction; | A 78% B 22% |

| Conditions | Yield |
|---|---|
| With Fe2(4,4″-dioxido-[1,1′:4′,1″-terphenyl]-3,3″-dicarboxylate); 1-(tert-butylsulfonyl)-2-iodosylbenzene In [D3]acetonitrile at 20℃; for 1.5h; | A 100% B 100% |
| With 3-chloro-benzenecarboperoxoic acid; [Ni2(L2H2)(OAc)2] at 20℃; for 1h; | A 7% B 93% |
| With 3-chloro-benzenecarboperoxoic acid; (5,10,15,20-tetrakis(pentafluorophenyl)porphyrinato)iron(III) chloride In dichloromethane; acetonitrile for 1h; Product distribution; Ambient temperature; other catalysts; kinetic isotope effect; | A 2% B 89% |
-
-
110-83-8
cyclohexene

-
A
-
930-68-7
cyclohexenone

-
B
-
286-20-4
cyclohexane-1,2-epoxide

-
C
-
108-94-1
cyclohexanone

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In isopropyl alcohol at 50℃; for 15h; Further byproducts given; | A 25.9% B 3.3% C 2.3% D 4.1% |
| With hydrogen; oxygen at 100℃; | A 0.09% B 0.03% C 0.02% D 0.01% |
| With tert.-butylhydroperoxide In water at 70 - 80℃; under 760.051 Torr; for 6h; Catalytic behavior; | |
| With tert.-butylhydroperoxide In water at 70 - 80℃; under 760.051 Torr; for 6h; Catalytic behavior; |


| Conditions | Yield |
|---|---|
| With hydrogen In Hexadecane at 200℃; under 7600.51 Torr; for 1h; Reagent/catalyst; Autoclave; | A 28.8 %Chromat. B 23.8 %Chromat. |
| With hydrogen In Hexadecane at 200℃; under 7600.51 Torr; for 1h; Reagent/catalyst; Autoclave; | A 31.2 %Chromat. B 35 %Chromat. |
| With hydrogen In Hexadecane at 200℃; under 7600.51 Torr; for 1h; Reagent/catalyst; Autoclave; |

| Conditions | Yield |
|---|---|
| With water In aq. phosphate buffer at 25℃; for 1.5h; pH=2.7 - 3.2; Electrochemical reaction; | A 33% B 66% |

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With urea hydrogen peroxide adduct In [D3]acetonitrile at 20℃; pH=8; | 84% |
| With urea hydrogen peroxide adduct In aq. phosphate buffer; [D3]acetonitrile; water-d2 at 20℃; for 0.0333333h; pH=8; | 84 %Spectr. |
-
-
98-01-1
furfural

-
A
-
97-99-4
Tetrahydrofurfuryl alcohol

-
B
-
534-22-5
2-methylfuran

-
C
-
98-00-0
(2-furyl)methyl alcohol

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With Pd catalyst supported on CMK-5 mesoporous carbon In isopropyl alcohol at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 16.8% B 8.9% C 34% D 21.1% |
| With Pd catalyst supported on CMK-5 mesoporous carbon In isopropyl alcohol at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 31.5% B 13.4% C 20.3% D 16.3% |

-
-
98-01-1
furfural

-
A
-
97-99-4
Tetrahydrofurfuryl alcohol

-
B
-
98-00-0
(2-furyl)methyl alcohol

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With Pd catalyst supported on Vulcan In isopropyl alcohol at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 7.2% B 24.3% C 11.6% |
| With Pd catalyst supported on MSU-F-C mesoporous carbon In isopropyl alcohol at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 9.4% B 19.8% C 23.1% |


| Conditions | Yield |
|---|---|
| With hydrogen In dodecane at 200℃; under 15001.5 Torr; for 2h; | A 5.2% B 62.4% |
| With ethanol; platinum at 20℃; Hydrogenolyse; | |
| With dihydrogen hexachloroplatinate; hydrogen; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate; 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide at 60℃; under 7500.75 Torr; for 15h; Autoclave; |
-
-
100-66-3
methoxybenzene

-
A
-
931-56-6
2-methoxycyclohexane

-
B
-
110-82-7
cyclohexane

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen In decalin at 20 - 220℃; under 37503.8 - 45004.5 Torr; Inert atmosphere; Autoclave; | A 72% B 12% C n/a |
| With 5% active carbon-supported ruthenium; hydrogen; magnesium oxide In water at 159.84℃; under 11251.1 Torr; for 2h; | A 69% B 9% C 21% |
| With hydrogen In Hexadecane at 280℃; under 36003.6 Torr; for 6h; Catalytic behavior; Kinetics; | A 35.38% B 7.51% C 5.52% |

-
-
18027-46-8
cyclohexyloxy(triethoxy)silane

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In tetrahydrofuran; methanol at 50℃; for 48h; | |
| With water; sodium hydroxide In methanol | |
| With sodium hydroxide In methanol at 60℃; for 24h; Inert atmosphere; Schlenk technique; |

| Conditions | Yield |
|---|---|
| With 5 wt% ruthenium/carbon; hydrogen In water at 199.84℃; under 15001.5 Torr; for 4h; Autoclave; | |
| With hydrogen In water at 230℃; under 7500.75 Torr; for 4h; Reagent/catalyst; Autoclave; |

| Conditions | Yield |
|---|---|
| With water In aq. phosphate buffer at 25℃; for 1.5h; pH=2.7 - 3.2; Electrochemical reaction; | A 40% B 57% |

| Conditions | Yield |
|---|---|
| With lithium borohydride; 9-methoxy-9-BBN In diethyl ether at 25℃; for 0.25h; Product distribution; | 100% |
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 6h; | 100% |
| With LiCrH4*2LiCl*2THF In tetrahydrofuran at 25℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol for 1h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With tetrachlorosilane; sodium iodide In dichloromethane; acetonitrile for 4h; Heating; | 100% |
| With phenylthiotrimethylsilane; tetra-(n-butyl)ammonium iodide; zinc(II) iodide In 1,2-dichloro-ethane at 60℃; for 8h; | 97 % Chromat. |
-
-
20445-33-4, 39637-99-5
(R)-methoxytrifluoromethylphenylacetyl chloride

-
-
108-94-1
cyclohexanone

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With bis(phosphine)rhodium; coimmobilized horse liver alcohol dehydrogenase and D- and L-lactate dehydrogenase; NAD; sodium lactate In water for 192h; pH 8.0; | 100% |

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With water | 100% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine | 100% |
| With triphenylphosphine | |
| With triphenylphosphine In diethyl ether | |
| With triphenylphosphine |
-
-
930-68-7
cyclohexenone

-
A
-
502-44-3
hexahydro-2H-oxepin-2-one

-
B
-
108-94-1
cyclohexanone

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With Geotrichum candidum CCT 1205 whole cells In aq. phosphate buffer at 28℃; for 24h; pH=6.5; Baeyer-Villiger Ketone Oxidation; Enzymatic reaction; | A n/a B 100% C n/a |


| Conditions | Yield |
|---|---|
| With Ni0.85Rh0.15; hydrogen In water at 95℃; under 760.051 Torr; for 16h; Reagent/catalyst; | A 100% B 5% C 79% |
| With isopropyl alcohol at 150℃; under 7500.75 Torr; for 48h; Inert atmosphere; Autoclave; | A 100% B 12.2% C 87.5% |
| With isopropyl alcohol at 150℃; under 7500.75 Torr; for 3h; Catalytic behavior; Temperature; Inert atmosphere; Autoclave; | A 100% B 73.1% C 22.4% |
| With Ru0.6Ni0.4; hydrogen In water at 95℃; under 760.051 Torr; for 16h; Reagent/catalyst; | A 98% B 61% C 6% |
| With hydrogen In n-heptane at 140℃; under 750.075 Torr; for 6h; Catalytic behavior; | A 34 %Chromat. B 12 %Chromat. C 22 %Chromat. |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol at 150℃; under 7500.75 Torr; for 12h; Inert atmosphere; Autoclave; | A 25.1% B 100% C 74.9% |
| With isopropyl alcohol at 160℃; for 15h; Autoclave; Inert atmosphere; | |
| With isopropyl alcohol at 150℃; for 10h; Catalytic behavior; Reagent/catalyst; Temperature; Sealed tube; | A 24.6 %Chromat. B 47.8 %Chromat. C 24.3 %Chromat. |
| With isopropyl alcohol at 150℃; for 6h; Temperature; Sealed tube; | A 15.2 %Chromat. B 17.7 %Chromat. C 5.8 %Chromat. |


| Conditions | Yield |
|---|---|
| With methanol; zirconium(IV) chloride at 20℃; for 5h; | 99% |
| silica-supported prop-1-ylsulfonic acid In methanol | 99.1% |
| Nafion-H In methanol for 3h; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 160℃; for 10h; Temperature; Concentration; Reagent/catalyst; | 99% |
| With potassium hydroxide; Raney Ni-Al; water at 90℃; for 16h; | 90.8% |
| With acetic acid; platinum Hydrogenation; |
-
-
14289-72-6
allyl cyclohexyl carbonate

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With Fe3O4@SiO2-[(4-(5-O3Si-pentylcarbamoyl)-2-pyridinecarboxylato)CpRu(η3-C3H5)]PF6 In methanol at 30℃; for 1h; Inert atmosphere; chemoselective reaction; | 99% |
| With diethylamine; palladium diacetate; trisodium tris(3-sulfophenyl)phosphine In water; acetonitrile for 0.166667h; Ambient temperature; | 80% |
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 0.5h; | 99 % Spectr. |

| Conditions | Yield |
|---|---|
| With sodium thiophenolate; thiophenol In acetonitrile for 2h; Product distribution; Substitution; elimination; | A 5.1% B 94% C 99% |


| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); cetyltrimethylammonim bromide; lithium tri-t-butoxyaluminum hydride; sodium t-butanolate; tricyclohexylphosphine In toluene at 70℃; for 5h; Micellar solution; | A 99% B 99% |
| With Ru0.6Ni0.4; hydrogen In water at 95℃; under 760.051 Torr; for 16h; Reagent/catalyst; | A 92% B 96% |
| With hydrogen In water at 110℃; under 7500.75 Torr; for 1h; Autoclave; |
-
-
946-80-5
(benzyloxy)benzene

-
A
-
82166-21-0
methyl cyclohexane

-
B
-
108-88-3
toluene

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol at 120℃; for 2h; | A 28% B 72% C 99% |
| With Rh0.6Ni0.4; hydrogen In water at 95℃; under 760.051 Torr; for 16h; Reagent/catalyst; | A 35% B 63% C 95% |
| With 10% Pd/C; hydrogen In hexane at 160℃; under 30003 Torr; for 2h; Autoclave; | |
| With isopropyl alcohol at 170℃; for 15h; Sealed tube; | A 0.68 mmol B 0.28 mmol C 0.94 mmol |

-
-
40515-89-7
2-phenethoxybenzene

-
A
-
1678-91-7
ethyl-cyclohexane

-
B
-
100-41-4
ethylbenzene

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen; lanthanum(lll) triflate In isopropyl alcohol at 120℃; for 2h; | A 33% B 66% C 99% |
| With hydrogen In n-heptane at 160℃; under 750.075 Torr; for 6h; Catalytic behavior; Temperature; | A 8 %Chromat. B 92 %Chromat. C 100 %Chromat. |
| With isopropyl alcohol at 170℃; under 7500.75 Torr; Inert atmosphere; Autoclave; |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; Nd(EBAB)Cl3 In methanol for 1h; Product distribution; Ambient temperature; other reagents: Ln(EBAB)Cl3, Ce(EBAB)Cl3, Pr(EBAB)Cl3, Sm(PBAB)Cl3, Yb(PBAB)Cl3, Lu(PBAB)Cl3; | A 98.5% B 1.5% |
| With N-tert-butylaminoborane In diethyl ether for 16h; Ambient temperature; Yields of byproduct given; | A 87% B n/a |
| With potassium hydroxide; hydrogen; RuCl2<(R)-binap>(dmf)n*(R,R)-1,2-diphenylethylenediamine In isopropyl alcohol at 28℃; under 3040 Torr; for 1h; | A 65% B 2% |

| Conditions | Yield |
|---|---|
| With nickel dichloride; RedAl In tetrahydrofuran at 68℃; for 2h; Product distribution; other dehalogenating systems, other reaction times and temperatures; | A n/a B 98% |
-
-
13871-89-1
trimethylsilyl cyclohexyl ether

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With (Ppyz)Zr(BH4)2Cl2 In diethyl ether at 20℃; for 9h; | 98% |
| With Oxone In methanol for 0.4h; Heating; | 96% |
| With iron(III) chloride In acetonitrile for 0.0166667h; Product distribution; Ambient temperature; var. Lewis acids; other silyl ethers; | 95% |
-
-
80866-33-7
cis-2-(trimethylsilyl)cyclohexyl trifluoroacetate

-
A
-
110-83-8
cyclohexene

-
B
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| In ethanol at 65℃; Kinetics; ΔH, ΔG, ΔS (excit.); | A 98% B n/a |
| In water at 60℃; Kinetics; | A 9.2% B n/a |

| Conditions | Yield |
|---|---|
| With borane-ammonia complex; Pd(SIPr)(PCy3) In isopropyl alcohol at 50℃; for 16h; Inert atmosphere; Glovebox; | 97% |
| With formic acid; C18H24ClIrN3 In water at 80℃; for 4h; Schlenk technique; Inert atmosphere; | 94% |
| With formic acid; C18H14ClN4O2Ru(1+)*Cl(1-); sodium formate In water at 60℃; for 20h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 80℃; under 7500.75 Torr; for 20h; Autoclave; | 97% |
| With 1,1'-bis(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; Butane-1,4-diol; potassium tert-butylate at 110℃; for 24h; Inert atmosphere; | 68% |
| With sodium tetrahydroborate; C11H18Cl2CoN2S; hydrogen In isopropyl alcohol at 100℃; under 37503.8 Torr; for 16h; Glovebox; Autoclave; | 47% |

| Conditions | Yield |
|---|---|
| With ammonium chloride In methanol Electrochemical reaction; | A 96% B 1% C 1% |
| With {(η6-C6H6)Ru(NCCH3)3}{BF4}2; water; hydrogen In benzene at 110℃; under 30400 Torr; for 4h; | A 68% B 1% C 2% |
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane at -78℃; for 3h; Yield given. Yields of byproduct given; |

-
-
108-94-1
cyclohexanone

-
-
124-40-3
dimethyl amine

-
A
-
100-60-7
N-methylcyclohexylamine

-
B
-
98-94-2
N,N-dimethyl-cyclohexanamine

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen at 160 - 220℃; under 67506.8 - 97509.8 Torr; for 100 - 1000h; Product distribution / selectivity; | A 0.1% B 96% C 0.5% |
| With hydrogen at 160 - 180℃; under 63756.4 - 97509.8 Torr; for 100 - 400h; Product distribution / selectivity; | A 0.1% B 94% C 2% |


| Conditions | Yield |
|---|---|
| With iodine for 0.333333h; Ambient temperature; | 100% |
| With SBA-15-Ph-Pr-SO3H at 20℃; for 0.5h; | 100% |
| yttria-stabilized zirconia In acetonitrile for 4h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| zirconium(IV) oxide at 200℃; Heating; reflux, 5 h in liquid-phase; var. temp.: 130 deg C; | 100% |
| With β-cyclodextrin-SO3H In neat (no solvent) at 70℃; for 5h; | 99% |
| LaY zeolite at 116℃; for 10h; Acetylation; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In benzene for 2.5h; Fischer esterification; Heating; | 100% |
| With sulfuric acid In chloroform for 5h; Reflux; | 78% |
| With sulfuric acid In toluene for 24h; Reflux; | 77% |
| With hydrocarbon; toluene-4-sulfonic acid unter Entfernung des entstehenden Wassers durch azeotrope Destillation; |
-
-
2524-64-3
chlorophosphoric acid diphenyl ester

-
-
108-93-0
cyclohexanol

-
-
4281-67-8
cyclohexyl diphenyl phosphate

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylamine In dichloromethane at 20℃; for 1h; | 100% |
| With titanium tetrachloride; triethylamine In tetrahydrofuran at 20℃; for 1h; | 97% |
| With pyridine N-oxide; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In dichloromethane at 20℃; for 8h; Inert atmosphere; | 94% |
-
-
108-93-0
cyclohexanol

-
-
98-89-5
Cyclohexanecarboxylic acid

-
-
15840-96-7
cyclohexyl cyclohexanecarboxylate

| Conditions | Yield |
|---|---|
| zirconium(IV) oxide at 210℃; for 2h; in autoclave; 5 h, reflux in liquid-phase; | 100% |
| With dmap; iodine; di-2-thienyl carbonate In acetonitrile at 20℃; for 0.5h; | 89% |
| With dmap; 2-methyl-6-nitrobenzoic anhydride; triethylamine In dichloromethane at 20℃; for 20h; | 87% |

| Conditions | Yield |
|---|---|
| With oxygen; sodium nitrite In trifluoroacetic acid at 0 - 20℃; for 5h; | 100% |
| With oxygen; potassium nitrate; trifluoroacetic acid at 0 - 20℃; for 5.25h; Product distribution / selectivity; | 100% |
| With potassium nitrite; oxygen; trifluoroacetic acid at 0 - 20℃; for 5.25h; Product distribution / selectivity; | 100% |
Cyclohexanol Consensus Reports
Cyclohexanol Standards and Recommendations
ACGIH TLV: TWA 50 ppm (skin)
DFG MAK: 50 ppm (210 mg/m3)
Cyclohexanol Analytical Methods
Cyclohexanol Specification
This chemical has the IUPAC name Cyclohexanol, and it's also named as 1-cyclohexanol. With the CAS registry number 108-93-0, its product categories are Essential Chemicals; Reagent Plus; Routine Reagents; Alcohols; C2 to C6; Oxygen Compounds and its classification codes are Human Data; Mutation data; Reproductive Effect; Skin / Eye Irritant; TSCA Flag T [Subject to the Section 4 test rule under TSCA]. However, this chemical is an alcohol and it should be stored in the cool and dry place. Keep it away from the oxides, heat, fire and acid.
Other characteristics of the Cyclohexanol can be summarised as followings: (1)ACD/LogP: 1.28; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.282; (4)ACD/LogD (pH 7.4): 1.282; (5)ACD/BCF (pH 5.5): 5.551; (6)ACD/BCF (pH 7.4): 5.551; (7)ACD/KOC (pH 5.5): 118.699; (8)ACD/KOC (pH 7.4): 118.699; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.478; (14)Molar Refractivity: 29.261 cm3; (15)Molar Volume: 103.441 cm3; (16)Polarizability: 11.6×10-24cm3; (17)Surface Tension: 32.496 dyne/cm; (18)Density: 0.968 g/cm3; (19)Flash Point: 67.778 °C; (20)Enthalpy of Vaporization: 46.14 kJ/mol; (21)Boiling Point: 159.552 °C at 760 mmHg; (22)Vapour Pressure: 0.876 mmHg at 25°C.
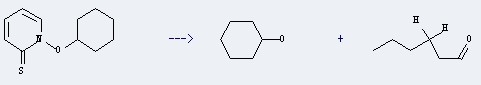
The Cyclohexanol could be obtained by the method of phenol hydrogenation and cyclohexane oxidation: It could be made by the reactant of 1-cyclohexyloxy-1H-pyridine-2-thione. This reaction needs the reagents of tributylstannane, AIBN, and the solvent of benzene. The yield is 73 %. In addition, this reaction should be taken for 20 minutes at the temperature of 80 °C.
Uses of the Cyclohexanol: It's mainly used for the production of adipic acid, hexamethylene diamine, cyclohexanone, caprolactam and as a soap stabilizing agent, disinfection medicated soap and detergent emulsion. It's also used as solvents such as rubber, resins, nitrocellulose, metallic soaps, oils, esters, ethers. Paint blending agent, leather degreasing agent, release agent, dry cleaners, polishes are also made from the material of this chemical.
When you are using this chemical, please be cautious about it as the following: This chemical is harmful by inhalation and if swallowed. It's irritating to respiratory system and skin. Please avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
1.SMILES: C1CCC(CC1)O
2.InChI: InChI=1/C6H12O/c7-6-4-2-1-3-5-6/h6-7H,1-5H2
3.InChIKey: HPXRVTGHNJAIIH-UHFFFAOYAN
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| frog | LDLo | parenteral | 1914mg/kg (1914mg/kg) | CARDIAC: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 24, Pg. 405, 1925. |
| human | TCLo | inhalation | 75ppm (75ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 282, 1943. |
| mouse | LD50 | intramuscular | 1gm/kg (1000mg/kg) | Journal of Scientific and Industrial Research, Section C: Biological Sciences. Vol. 21, Pg. 342, 1962. | |
| mouse | LD50 | intraperitoneal | 1352mg/kg (1352mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 1254, 1969. | |
| mouse | LD50 | intravenous | 272mg/kg (272mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 135, Pg. 330, 1962. | |
| rabbit | LDLo | oral | 2200mg/kg (2200mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 199, 1943. |
| rabbit | LDLo | skin | 794mg/kg (794mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LIVER: CHANGE IN GALL BLADDER STRUCTURE OR FUNCTION | National Technical Information Service. Vol. OTS0538617, |
| rat | LC | inhalation | > 6500mg/m3/1H (6500mg/m3) | National Technical Information Service. Vol. OTS0572832, | |
| rat | LD50 | oral | 1400mg/kg (1400mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0538617, |
Related Products
- Cyclohexanol
- Cyclohexanol, 1-propyl-
- Cyclohexanol, 2-chloro-
- Cyclohexanol, 4-(2-amino-1H-benzimidazol-1-yl)-
- Cyclohexanol, 4-(dimethylamino)-
- Cyclohexanol, 4-butyl-
- Cyclohexanol, 4-chloro-
- Cyclohexanol, 4-ethyl-,trans-
- Cyclohexanol,1-(2-propen-1-yl)-
- Cyclohexanol,1-(aminomethyl)-
- 108930-00-3
- 108934-21-0
- 108936-90-9
- 108937-85-5
- 108938-16-5
- 1089-40-3
- 108-94-1
- 108-95-2
- 108957-20-6
- 108957-72-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View