-
Name
DL-Mevalonolactone
- EINECS 211-615-0
- CAS No. 674-26-0
- Article Data42
- CAS DataBase
- Density 1.189 g/cm3
- Solubility Soluble in ethanol (100 mg/ml-clear to very slightly hazy faint yellow solution), dichloromethane, ethyl acetate, and methanol
- Melting Point 28 °C(lit.)
- Formula C6H10 O3
- Boiling Point 145-150 °C5 mm Hg(lit.)
- Molecular Weight 130.144
- Flash Point >230 °F
- Transport Information
- Appearance Moist pale yellow to off white solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2H-Pyran-2-one,tetrahydro-4-hydroxy-4-methyl-, (?à)- (8CI); (RS)-Mevalonolactone; (?à)-Mevalolactone; (?à)-Mevalonolactone; DL-Mevalonic acid lactone;DL-Mevalonic acid d-lactone; DL-Mevalonolactone; MVSL; Mevalolactone; Mevalonic acid lactone;Mevalonic acid d-lactone;Mevalonic lactone; Mevalonolactone; NSC 90804; b-Hydroxy-b-methyl-d-valerolactone
- PSA 46.53000
- LogP 0.07440
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In dichloromethane for 4h; Ambient temperature; | 92% |
| With aluminum oxide; sodium bromite In dichloromethane for 1.5h; Ambient temperature; | 85% |
| With peracetic acid; sodium bromide In ethyl acetate at 39.9℃; for 2h; | 83% |
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate In acetonitrile at 22℃; for 6h; | 83% |
-
-
118617-84-8
4-(4-Methoxy-benzyloxymethoxy)-4-methyl-tetrahydro-pyran-2-one

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water for 10h; Ambient temperature; cleavage of (p-methoxybenzyloxy)methyl protecting group; | 89% |
-
-
99720-12-4
2,3,5,6-tetrahydro-4-methylpyran-2,4-diol

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sodium acetate; pyridinium chlorochromate In dichloromethane for 6h; Ambient temperature; | 82% |
-
-
150-97-0, 690-72-2, 17817-88-8, 32451-23-3
D,L-mevalonic acid

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sulfuric acid for 15h; Reflux; | 82% |
| Multi-step reaction with 2 steps 1: tetrabutylammomium bromide / tetrahydrofuran / 4 h / 50 °C / Inert atmosphere 2: pyridine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere View Scheme |
-
-
2754-27-0
trimethylsilyl acetate

-
-
81852-67-7
4-((trimethylsilyl)oxy)-3-butanone

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide 1.) THF, -78 deg C, 2 h, 2.) -78 deg C, 2 h; | 81% |
-
-
80514-97-2
ethyl 3-hydroxy-3-methyl-5-<(2-tetrahydropyranyl)-oxy>pentanoate

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With acetyl chloride In ethanol for 10h; Ambient temperature; | 80% |
| With acetyl chloride In ethanol Ambient temperature; overnight; | 22 g |
-
-
34695-32-4
3-hydroxy-3-methylglutaric anhydride

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In isopropyl alcohol | 75.6% |
| With sodium tetrahydroborate In isopropyl alcohol Ambient temperature; | 300 mg |
| With sodium tetrahydroborate In isopropyl alcohol for 24h; Ambient temperature; | |
| Multi-step reaction with 2 steps 1: 1.) CH2Cl2, -20 deg C, 7 days 2: 1.) LiCl, NaBH4; 2.) 10percent KOH / 1.) EtOH, 50 deg C, 24 h; 2.) MeOH, 60 deg C, 4h View Scheme |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
1452161-92-0
benzyl 3,5-dihydroxy-3-methylpentanoate

-
A
-
1452161-93-1
benzyl 2-(4-methyl-2-oxo-1,3-dioxan-4-yl)acetate

-
B
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; | A 64% B 12% |

-
-
128811-87-0
5-chloro-3-hydroxy-3-methylvaleric acid

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With potassium carbonate; Katamin AB In acetone at 40 - 45℃; for 3h; | 60% |
-
-
246237-03-6
5-[(4-nitrophenyl)methylcarbamoyloxy]-3-hydroxy-3-methylpentanoic acid

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; Cyclization; Deprotection; | 17% |

| Conditions | Yield |
|---|---|
| With diethyl ether; boron trifluoride behandeln des Reaktionsprodukts mit methanol. Kalilauge und anschliessend mit Chlorwasserstoff enthaltendem Methanol; |

| Conditions | Yield |
|---|---|
| With ozone Behandeln des Reaktionsgemisches mit Natriumboranat in Aethanol und Erhitzen des Reaktionsprodukts mit Aluminiumisopropylat in Xylol; |

| Conditions | Yield |
|---|---|
| With ozone anschliessend mit wss.Wasserstoffperoxid; |
-
-
87894-65-3
3-acetoxy-3-methylpentane-1,5-dioic acid anhydride

-
A
-
2381-87-5
4-methyl-5,6-dihydro-2H-pyran-2-one

-
B
-
78501-60-7
5-O-acetylmevalonic acid

-
C
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In isopropyl alcohol for 24h; Ambient temperature; Title compound not separated from byproducts; |

-
-
69739-34-0
t-butyldimethylsiyl triflate

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
120388-51-4
butyl <2-13C,2H3>acetate

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| 0.82 g |

-
-
24394-14-7, 127516-44-3, 128821-87-4
(+/-)-Phaseic acid

-
B
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With borane In tetrahydrofuran 1.) -18 deg C, 30 min, 2.) room temp.; | A 1.0 mg B 0.6 mg |
-
-
94726-38-2, 106402-01-1
3-Hydroxy-3-methyl-4-(2'-morpholin-4-yl-[1,1']binaphthalenyl-2-ylcarbamoyl)-butyric acid methyl ester

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium tetrahydroborate; lithium chloride 1.) EtOH, 50 deg C, 24 h; 2.) MeOH, 60 deg C, 4h; Yield given. Multistep reaction; |

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With barium dihydroxide; water at 120℃; Behandeln des erhaltenen Barium-Salzes mit wss.Schwefelsaeure und Erhitzen des Reaktionsprodukts; |

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol Erwaermen der erhaltenen, ueber das N,N'-Dibenzyl-aethylendiamin-Salz gereinigten (+-)-3,5-Dihydroxy-3-methyl-valeriansaeure unter 0.3 Torr; |

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With methanol; potassium hydroxide anschliessend mit Chlorwasserstoff enthaltendem Methanol; |

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| With hydrogenchloride Behandeln der mit wss.Natronlauge neutralisierten Reaktionsloesung mit Kaliumboranat und anschliessend mit wss.Salzsaeure; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; dihydrogen peroxide |
-
-
114580-75-5
(R/S)-3-methyl-5-hexene-1,3-diol

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 285 mg / DMAP; NEt3 / tetrahydrofuran; toluene / 90 h / 20 °C 2: 4-methylmorpholine N-oxide; OsO4 / CH2Cl2; H2O; 2-methyl-propan-2-ol / 0.75 h 3: 409.1 mg / Pb(OAc)4 / CH2Cl2; H2O; 2-methyl-propan-2-ol / 0.17 h 4: 77 percent / KMnO4 / diethyl ether; aq. phosphate buffer; 2-methyl-propan-2-ol / 0.08 h / 20 °C / pH 7.4 5: 17 percent / NaOH / methanol / 20 °C View Scheme |
-
-
246236-98-6
(4-nitrophenyl)methylcarbamic acid 3-hydroxy-3-methyl-5-oxopentyl ester

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 77 percent / KMnO4 / diethyl ether; aq. phosphate buffer; 2-methyl-propan-2-ol / 0.08 h / 20 °C / pH 7.4 2: 17 percent / NaOH / methanol / 20 °C View Scheme |
-
-
246237-00-3
methyl-(4-nitro-phenyl)-carbamic acid 3-hydroxy-3-methyl-hex-5-enyl ester

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 4-methylmorpholine N-oxide; OsO4 / CH2Cl2; H2O; 2-methyl-propan-2-ol / 0.75 h 2: 409.1 mg / Pb(OAc)4 / CH2Cl2; H2O; 2-methyl-propan-2-ol / 0.17 h 3: 77 percent / KMnO4 / diethyl ether; aq. phosphate buffer; 2-methyl-propan-2-ol / 0.08 h / 20 °C / pH 7.4 4: 17 percent / NaOH / methanol / 20 °C View Scheme |

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 409.1 mg / Pb(OAc)4 / CH2Cl2; H2O; 2-methyl-propan-2-ol / 0.17 h 2: 77 percent / KMnO4 / diethyl ether; aq. phosphate buffer; 2-methyl-propan-2-ol / 0.08 h / 20 °C / pH 7.4 3: 17 percent / NaOH / methanol / 20 °C View Scheme |
-
-
25201-40-5
1,1-diallylethanol

-
-
674-26-0
mevalonolactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) O3, 2.) H2O2 / 1.) CH2Cl2, CH3COOH, -78 deg C, 2.) CH3COOH 2: (CH3CO)2O / 14 h / Ambient temperature 3: 75.6 percent / NaBH4 / propan-2-ol View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) ozone, acetic acid, 2.) hydrogen peroxide / 1.) methylene dichloride, -78 deg C, 2.) water, reflux, 13 h 2: 673 mg / acetic anhydride / 72 h / 18 °C / further reagents, reaction conditions 3: 300 mg / sodium borohydride / propan-2-ol / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (CH3CO)2O / 14 h / Ambient temperature 2: 75.6 percent / NaBH4 / propan-2-ol View Scheme | |
| Multi-step reaction with 2 steps 1: 673 mg / acetic anhydride / 72 h / 18 °C / further reagents, reaction conditions 2: 300 mg / sodium borohydride / propan-2-ol / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 16 h / 0 - 20 °C 2: tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 1-methyl-1H-imidazole / acetonitrile / 6 h / 22 °C View Scheme |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
674-26-0
mevalonolactone

-
-
100689-33-6, 120445-02-5
4-Methyl-4-(tetrahydro-pyran-2-yloxy)-tetrahydro-pyran-2-one

| Conditions | Yield |
|---|---|
| triphenylphosphine hydrobromide In dichloromethane for 7h; Ambient temperature; | 96% |
| With lithium hexafluorophosphate In hexane at 0℃; for 4.5h; | 49% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 19h; Reflux; | 93% |
| With PPA | 89% |
| With toluene-4-sulfonic acid In benzene for 20h; Reflux; Dean-Stark; | 89% |
-
-
88023-78-3
para-methoxybenzyl chloride

-
-
674-26-0
mevalonolactone

-
-
118617-84-8
4-(4-Methoxy-benzyloxymethoxy)-4-methyl-tetrahydro-pyran-2-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 30h; Ambient temperature; | 84% |
-
-
3173-56-6
benzyl isothiocyanate

-
-
674-26-0
mevalonolactone

-
-
123034-94-6
Benzyl-carbamic acid 4-methyl-2-oxo-tetrahydro-pyran-4-yl ester

| Conditions | Yield |
|---|---|
| copper(l) chloride In N,N-dimethyl-formamide Ambient temperature; | 80% |

| Conditions | Yield |
|---|---|
| With nitric acid; sodium nitrite at 35 - 40℃; for 5h; | 76% |
| With nitric acid at 40℃; for 4h; Cooling with ice; | 57% |
-
-
132-32-1
9-ethyl-9H-carbazol-3-ylamine

-
-
674-26-0
mevalonolactone

-
-
1427185-29-2
N-(9-ethyl-9H-carbazol-3-yl)-3,5-dihydroxy-3-methylpentanamide

| Conditions | Yield |
|---|---|
| Stage #1: 9-ethyl-9H-carbazol-3-ylamine With trimethylaluminum In toluene at 10 - 35℃; for 0.5h; Stage #2: mevalonolactone In toluene at 0℃; for 4h; | 74% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium Tetrafluoroborate In 1,4-dioxane at 240℃; for 0.583333h; Microwave irradiation; | 73% |
-
-
100-39-0
benzyl bromide

-
-
674-26-0
mevalonolactone

-
-
1452161-92-0
benzyl 3,5-dihydroxy-3-methylpentanoate

| Conditions | Yield |
|---|---|
| Stage #1: mevalonolactone With water; potassium hydroxide at 20 - 40℃; for 1h; Stage #2: benzyl bromide With hydrogenchloride; tetrabutylammomium bromide In tetrahydrofuran; water at 20 - 50℃; for 4h; pH=7 - 8; | 69% |
-
-
1548-77-2
2,3,4,5,6-pentafluorobenzylamine

-
-
674-26-0
mevalonolactone

-
-
1452162-37-6
3,5-Dihydroxy-3-methyl-N-((perfluorophenyl)methyl)pentanamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; for 12h; Inert atmosphere; | 53% |
-
-
652-31-3
2,3,4,5,6-pentafluorobenzamide

-
-
674-26-0
mevalonolactone

-
-
1452162-37-6
3,5-Dihydroxy-3-methyl-N-((perfluorophenyl)methyl)pentanamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20 - 80℃; for 12h; Inert atmosphere; | 53% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 55℃; for 5h; Heating; | 47% |
-
-
674-26-0
mevalonolactone

-
-
105338-90-7
3,5-dihydroxy-3-methyl-valeraldehyde-(2,4-dinitro-phenylhydrazone)

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; lithium aluminium tetrahydride Erwaermen des Reaktionsprodukts mit <2,4-Dinitro-phenyl>-hydrazin und wss.-methanol. HCl; |
-
-
91-00-9
Benzhydrylamine

-
-
674-26-0
mevalonolactone

-
-
5973-99-9
(+/-)-3,5-dihydroxy-3-methyl-valeric acid benzhydrylamide

| Conditions | Yield |
|---|---|
| at 110℃; for 1h; |
-
-
674-26-0
mevalonolactone

-
-
74-88-4
methyl iodide

-
-
10371-61-6
3,5-dihydroxy-3-methylpentanoic acid methyl ester

| Conditions | Yield |
|---|---|
| (i) ion-exchange resin + form>, H2O, (ii) Ag2O, (iii) /BRN= 969135/, THF; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran | |
| With lithium aluminium tetrahydride In tetrahydrofuran for 18h; Reflux; | 2.1 g |
-
-
3886-70-2
(R)-1-(1-Naphthyl)ethylamine

-
-
20445-33-4, 39637-99-5
(R)-methoxytrifluoromethylphenylacetyl chloride

-
-
674-26-0
mevalonolactone

-
-
72165-50-5, 72203-59-9
(R)-3,3,3-Trifluoro-2-methoxy-2-phenyl-propionic acid 3-hydroxy-3-methyl-4-((R)-1-naphthalen-1-yl-ethylcarbamoyl)-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine 1.) 70- 80 deg C, 3 h, 2.) RT; Multistep reaction; |


| Conditions | Yield |
|---|---|
| With arsenite rat liver extracts, phosphate buffer, pH 7.4, sucrose; |
DL-Mevalonolactone Chemical Properties
Product Name: DL-Mevalonolactone (CAS NO.674-26-0)
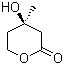
Molecular Formula: C6H10O3
Molecular Weight: 130.14g/mol
Mol File: 674-26-0.mol
EINECS: 211-615-0
Melting Point: 28 °C(lit.)
Boiling point: 274.9 °C at 760 mmHg
Storage Temperature: 2-8°C
Flash Point: 126.8 °C
Density: 1.189 g/cm3
Refractive index: n20/D 1.473(lit.)
Index of Refraction: 1.478 Molar Refractivity: 30.99 cm3
Molar Volume: 109.4 cm3
Surface Tension: 42.5 dyne/cm
Enthalpy of Vaporization: 59.6 kJ/mol
Vapour Pressure: 0.000662 mmHg at 25°C
XLogP3-AA: -0.3
H-Bond Donor: 1
H-Bond Acceptor: 3
Structure Descriptors of DL-Mevalonolactone (CAS NO.674-26-0):
IUPAC Name: 4-hydroxy-4-methyloxan-2-one
Canonical SMILES: CC1(CCOC(=O)C1)O
InChI: InChI=1S/C6H10O3/c1-6(8)2-3-9-5(7)4-6/h8H,2-4H2,1H3
InChIKey: JYVXNLLUYHCIIH-UHFFFAOYSA-N
Product Categories: Pale Yellow OilHeterocycles; Metabolites & Impurities
DL-Mevalonolactone Safety Profile
Safety Information of DL-Mevalonolactone (CAS NO.674-26-0):
WGK Germany: 3
F: 3-10
DL-Mevalonolactone Specification
DL-Mevalonolactone , its CAS NO. is 674-26-0, the synonyms are DL-Mevalonic acid lactone ; (4R)-4-Hydroxy-4-methyl-oxan-2-one ; (+/-)-Tetrahydro-4-hydroxy-4-methyl-2H-pyran-2-one .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View