-
Name
Diethyl (2-oxopropyl)phosphonate
- EINECS 682-094-9
- CAS No. 1067-71-6
- Article Data87
- CAS DataBase
- Density 1.09 g/cm3
- Solubility
- Melting Point
- Formula C7H15O4P
- Boiling Point 263.1 °C at 760 mmHg
- Molecular Weight 194.167
- Flash Point 126.9 °C
- Transport Information
- Appearance CLEAR COLORLESS TO LIGHT YELLOW LIQUID
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Phosphonicacid, (2-oxopropyl)-, diethyl ester (9CI);Phosphonic acid, acetonyl-, diethylester (6CI,7CI,8CI);1-Diethoxyphosphinyl-2-propanone;2-Oxopropylphosphonicacid diethyl ester;Diethyl(acetylmethyl)phosphonate;Diethyl 2-oxopropanephosphonate;Diethylacetonylphosphonate;Diethyl phosphonoacetone;NSC 408852;
- PSA 62.41000
- LogP 1.84150
Synthetic route

| Conditions | Yield |
|---|---|
| for 2h; Reflux; | 100% |
| at 90℃; for 10h; | 68% |
| at 90 - 160℃; |
-
-
389827-60-5
[2-(Methoxycarbonyl-hydrazono)-propyl]-phosphonic acid diethyl ester

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetone for 3h; Ambient temperature; | 94% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
598-31-2
1-bromoacetone

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With C30H36B10CuO4P2; potassium carbonate at 20℃; for 7h; Time; Reagent/catalyst; | 93% |

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 91% |
-
-
51533-70-1
diethyl (2-methylallyl)phosphonate

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dimethylsulfide; ozone In dichloromethane Oxidation; | 88% |
-
-
79-20-9
acetic acid methyl ester

-
-
683-08-9
Diethyl methylphosphonate

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0℃; for 4.5h; | 87% |
| With n-butyllithium In tetrahydrofuran at -78 - 20℃; |

| Conditions | Yield |
|---|---|
| Stage #1: chloroacetone With potassium iodide In acetone at 20℃; for 2h; Stage #2: triethyl phosphite In diethyl ether; acetone for 2h; Reflux; | 85% |
| With potassium iodide In acetone; acetonitrile at 20 - 50℃; for 10h; | 83% |
| With sodium iodide In acetonitrile at 20℃; | 51% |
-
-
151026-96-9
diethyl 1-methylthio-2-oxo-n-propylphosphonate

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 1h; Heating; | 81% |

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With oxalic acid; silica gel In dichloromethane for 1h; Ambient temperature; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; oxygen; palladium dichloride at 85℃; for 20h; | 80% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; lithium diisopropyl amide In tetrahydrofuran; hexane at -60℃; | 78% |
-
-
2466-76-4
N-Acetylimidazole

-
-
3095-95-2
diethylphosphonoacetic acid

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With triethylamine; magnesium chloride In acetonitrile at 20℃; for 24h; Acylation; | 77% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
123-54-6
acetylacetone

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Schlenk technique; chemoselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| In diethyl ether Arbuzov reaction; | 75% |
-
-
98732-76-4
E-1-Methyl-3-oxo-1-pentenylphosphonsaeurediethylester

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium carbonate In methanol; water for 30h; Ambient temperature; | A 6.9% B 74% |
-
-
98732-74-2
E-1-Methyl-3-oxo-1-butenylphosphonsaeurediethylester

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium carbonate In methanol; water at 20℃; for 48h; | A 8.2% B 72% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water at 85℃; Inert atmosphere; Schlenk technique; chemoselective reaction; | 72% |
-
-
78-95-5
chloroacetone

-
-
122-52-1
triethyl phosphite

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
B
-
5954-28-9
phosphoric acid diethyl ester isopropenyl ester

| Conditions | Yield |
|---|---|
| With potassium iodide In acetone; acetonitrile at 0℃; for 12h; Michaelis-Arbuzov reaction; | A 70% B n/a |

-
-
148599-29-5
2-methyl-2-phenylsulphonyloxirane

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| other substituted arylsulfonyl epoxides; multistep reaction: synthesis of β-keto phosphonates; | 70% |
| With sodium hydride 1) THF, mineral oil, rt, 10 min, 2) THF, rt, 30 min; Yield given. Multistep reaction; |
-
-
111-34-2
-butyl vinyl ether

-
-
151026-96-9
diethyl 1-methylthio-2-oxo-n-propylphosphonate

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With n-butyl tin hydride; 2,2'-azobis(isobutyronitrile) In toluene for 1h; Heating; Yields of byproduct given; | A n/a B 70% C n/a |
| With n-butyl tin hydride; 2,2'-azobis(isobutyronitrile) In toluene for 1h; Heating; Yields of byproduct given; | A n/a B n/a C 37% |

-
-
141-97-9
ethyl acetoacetate

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 70% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
2235-46-3
N,N-diethyl-3-oxobutanamide

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 66% |
-
-
917-54-4
methyllithium

-
-
146036-63-7, 124050-38-0
2-(diethoxyphosphinyl)-2-fluoro-acetic acid chloride

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
B
-
124050-39-1
diethyl (1-fluoro-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; dimethylsulfide In tetrahydrofuran; diethyl ether 1.) from -78 deg C to -45 deg C; 2.) -45 deg C, 2.5 h; | A 20% B 62% |

-
-
146036-63-7, 124050-38-0
2-(diethoxyphosphinyl)-2-fluoro-acetic acid chloride

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
B
-
124050-39-1
diethyl (1-fluoro-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| at -78 - -45℃; for 3h; | A 20% B 62% |

-
-
2044-64-6
N,N-Dimethylacetoacetamid

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 62% |
-
-
5396-89-4
benzyl acetoacetate

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 60% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
123-54-6
acetylacetone

-
A
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 4h; Time; Inert atmosphere; Schlenk technique; chemoselective reaction; | A 58% B 20% |
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 0.5h; Time; Inert atmosphere; Schlenk technique; chemoselective reaction; | A 23% B 53% |

-
-
34170-81-5
diethyl (2-chloro-2-oxoethyl)phosphonate

-
-
917-54-4
methyllithium

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; dimethylsulfide In tetrahydrofuran; diethyl ether 1.) from -78 deg C to -45 deg C; 2.) -45 deg C, 2.5 h; | 55% |
-
-
34170-81-5
diethyl (2-chloro-2-oxoethyl)phosphonate

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether -78 deg C to -40 deg C; -40 deg C, 90 min; | 55% |

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With diethylamino-sulfur trifluoride In dichloromethane at -84 - 20℃; for 4h; | 51% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 20℃; for 15h; | 100% |
| With potassium carbonate In water at 20℃; for 18h; | 100% |
| With potassium carbonate In water at 20℃; for 18h; other aldehyde; | 100% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
68426-09-5
(4-methoxyphenoxy)acetaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 0 - 20℃; for 1h; Wadsworth-Emmonds-Horner reaction; | 100% |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
33046-41-2, 56161-62-7
(E)-6-phenyl-3-hexen-2-one

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl (2-oxopropyl)phosphonate With 1,8-diazabicyclo[5.4.0]undec-7-ene; lithium chloride In acetonitrile at 23℃; for 0.25h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; Stage #2: 3-phenyl-propionaldehyde In acetonitrile at 0 - 23℃; for 0.5h; Inert atmosphere; | 99% |
| With lithium hydroxide monohydrate In tetrahydrofuran at 0℃; for 2h; | 85% |
| Stage #1: Diethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran for 0.0833333h; Stage #2: 3-phenyl-propionaldehyde In tetrahydrofuran | 82% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
19790-60-4
3-Benzyloxypropanal

-
-
34591-98-5
(E)-6-(benzyloxy)hex-3-en-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane; water at 20℃; for 3h; Inert atmosphere; | 99% |
| With lithium hydroxide In tetrahydrofuran at 0 - 23℃; Horner-Wadsworth-Emmons reaction; |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
20511-04-0, 3815-31-4
(E)-4-(4-bromophenyl)but-3-ene-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane; water at 20℃; for 3h; Inert atmosphere; | 99% |
| Stage #1: Diethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 4-bromo-benzaldehyde In tetrahydrofuran at 0℃; for 2h; Horner-Emmons reaction; | 81% |
| With lithium hydroxide In tetrahydrofuran at 0 - 23℃; Horner-Wadsworth-Emmons reaction; | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran Horner-Emmons reaction; |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
94445-75-7
4-(pyridin-4-yl)-3-buten-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane; water at 20℃; for 3h; Inert atmosphere; | 99% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With RuCl2((R)-binap)(dmf)n; hydrogen In methanol at 50℃; under 3040 Torr; for 50h; | 98% |
| With baker's yeast In water at 28 - 30℃; for 120h; | 78% |
| With Geotrichum candidum IFO 4597 In water; isopropyl alcohol at 30℃; for 96h; | 72% |
| With (R)-TriMe-NAPhePHOS; hydrogen bromide; hydrogen; [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II) In ethanol at 50℃; under 7500.6 Torr; for 24h; | |
| With Rhodotorula rubra; chloroacetic acid ethyl ester |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
837364-61-1
Ethyl-{5-[3-formyl-1-(tetrahydro-pyran-2-yl)-1H-indazol-5-yl]-4-methyl-pyridin-3-ylmethyl}-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; water at 20℃; for 20h; | 98% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
21047-57-4
diethyl (1-diazo-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With 4-toluenesulfonyl azide; triethylamine In acetonitrile Inert atmosphere; | 97% |
| With 4-acetamidobenzenesulfonyl azide; triethylamine In acetonitrile at 0 - 20℃; | 95% |
| With 4-toluenesulfonyl azide; sodium hydride In tetrahydrofuran | 91% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
472965-44-9
4-(14-bromo-tetradec-2-ynyloxymethyl)-2,2-dimethyl-oxazolidine-3-carboxylic acid tert-butyl ester

-
-
472965-45-0
4-[17-(diethoxy-phosphoryl)-16-oxo-heptadec-2-ynyloxymethyl]-2,2-dimethyl-oxazolidine-3-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl (2-oxopropyl)phosphonate With n-butyllithium; sodium hydride In tetrahydrofuran at -78℃; Stage #2: 4-(14-bromo-tetradec-2-ynyloxymethyl)-2,2-dimethyl-oxazolidine-3-carboxylic acid tert-butyl ester In tetrahydrofuran Further stages.; | 97% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
3815-30-3
4-(4-methoxyphenyl)-3-buten-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane; water at 20℃; for 16h; Inert atmosphere; | 97% |
| With lithium hydroxide In tetrahydrofuran at 0 - 23℃; Horner-Wadsworth-Emmons reaction; |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
620-23-5
m-tolyl aldehyde

-
-
15753-84-1, 95416-57-2
(E)-4-(m-tolyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane; water at 20℃; for 3h; Inert atmosphere; | 97% |
| With potassium carbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; for 2h; Inert atmosphere; |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
34713-70-7
2-Phenylpropanal

-
-
60405-50-7
5-phenyl-3-hexene-2-one

| Conditions | Yield |
|---|---|
| barium dihydroxide In 1,4-dioxane at 70℃; for 0.166667h; Product distribution; further method; further 2-oxoalkanephosphonates; | 96% |
| barium dihydroxide In 1,4-dioxane at 70℃; for 0.166667h; | 96% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
86429-26-7
(+/-)-3,3-dimethyl-2-phenylbutanal

| Conditions | Yield |
|---|---|
| barium dihydroxide In 1,4-dioxane at 70℃; for 0.25h; | 96% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
1846-67-9
hept-2-ynal

-
-
63922-98-5
trans-3-decen-5-yn-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In water | 96% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile for 24h; Biginelli Pyrimidone Synthesis; Reflux; | 96% |
| With zinc trifluoromethanesulfonate In toluene for 3h; Reflux; Green chemistry; | 86% |
| ytterbium trifluoromethanesulfonate In toluene for 12h; Heating; | 58% |

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
20180-08-9
(R,S)-Diethyl-(2-hydroxypropyl)phosphonat

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol at 0℃; | 95% |
| With pyridine; sodium tetrahydroborate In ethanol 1.) -30 deg C, 10 min, 2.) r.t., 45 min; | 89% |
| With diethyl ether Irradiation; | |
| With sodium tetrahydroborate In ethanol for 2h; Reduction; | |
| With sodium tetrahydroborate In methanol at 0 - 20℃; |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
98934-28-2
diethoxyphosphinyl-3 N,N-dimethylamino-4 butene-3 one-2

| Conditions | Yield |
|---|---|
| With calcium chloride for 2h; Heating; | 95% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
109-73-9
N-butylamine

-
-
82101-95-9
N-[(1-methyl-2-diethoxyphosphoryl)ethyl]-N-n-butylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium cyanoborohydride In methanol for 4h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| at 130℃; for 8h; | 95% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
2158-14-7
4-acetamidobenzenesulfonyl azide

-
-
21047-57-4
diethyl (1-diazo-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 0 - 20℃; Inert atmosphere; | 95% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
28663-68-5
ethyl (phenylhydrazono)chloroacetate

-
-
81153-64-2
5-methyl-1-phenyl-1H-pyrazole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With lithium hydroxide In 1,2-dimethoxyethane at 20℃; for 10h; regioselective reaction; | 95% |
| With lithium hydroxide In 1,2-dimethoxyethane at 20℃; for 10h; regioselective reaction; | 95% |
| With lithium hydroxide In 1,2-dimethoxyethane at 20℃; for 10h; |

| Conditions | Yield |
|---|---|
| With sodium hydride | 94% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
147224-13-3
5-ethyl-hept-3-en-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 100℃; for 0.166667h; | 93% |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
111252-30-3
α-diethoxyphosphoryl-α,α-dichloroacetone

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In dichloromethane for 2h; | 93% |
| With chlorine In tetrachloromethane at 0℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With indium In tetrahydrofuran at 20℃; for 0.583333h; | 93% |
| With zinc 1.) THF, room temperature, 2.) THF, -78 deg C, 30 min; Yield given; Multistep reaction; |
-
-
1067-71-6
Diethyl (2-oxopropyl)phosphonate

-
-
357924-22-2
8,8-dimethyl-2-methylene-6-oxabicyclo[3.2.1]octan-7-ol

-
-
357924-23-3
1-(8,8-dimethyl-2-methylene-6-oxabicyclo[3.2.1]oct-7-yl)acetone

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl (2-oxopropyl)phosphonate With barium dihydroxide at 140℃; for 2h; Stage #2: 3-hydroxy-2,2-dimethyl-6-methylenecyclohexanecarbaldehyde; 8,8-dimethyl-2-methylene-6-oxabicyclo[3.2.1]octan-7-ol In tetrahydrofuran; water at 20℃; for 30h; Further stages.; | 92% |

Diethyl (2-oxopropyl)phosphonate Chemical Properties
Molecule structure of Phosphonic acid,P-(2-oxopropyl)-, diethyl ester (CAS NO.1067-71-6):
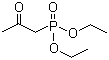
IUPAC Name: 1-Diethoxyphosphorylpropan-2-one
Molecular Weight: 194.165361 [g/mol]
Molecular Formula: C7H15O4P
Index of Refraction: 1.417
Molar Refractivity: 44.87 cm3
Molar Volume: 178 cm3
Surface Tension: 33.1 dyne/cm
Density: 1.09 g/cm3
Flash Point: 126.9 °C
Enthalpy of Vaporization: 50.09 kJ/mol
Melting Point: 126 °C at 9 mm Hg(lit.)
Boiling Point: 263.1 °C at 760 mmHg
Vapour Pressure: 0.0105 mmHg at 25 °C
H-Bond Acceptor: 4
Rotatable Bond Count: 6
Tautomer Count: 3
Exact Mass: 194.070795
MonoIsotopic Mass: 194.070795
Topological Polar Surface Area: 52.6
Heavy Atom Count: 12
Canonical SMILES: CCOP(=O)(CC(=O)C)OCC
InChI: InChI=1S/C7H15O4P/c1-4-10-12(9,11-5-2)6-7(3)8/h4-6H2,1-3H3
InChIKey: RSAFKRSMGOSHRK-UHFFFAOYSA-N
Product Categories: C-C Bond Formation; Horner-Wadsworth-Emmons Reagents; Olefination
Diethyl (2-oxopropyl)phosphonate Safety Profile
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
Diethyl (2-oxopropyl)phosphonate Specification
Phosphonic acid,P-(2-oxopropyl)-, diethyl ester (CAS NO.1067-71-6) is also called Diethyl acetonylphosphonate ; Diethyl (2-oxopropyl)phosphonate ; (2-Oxo-propyl)-phosphonicaciddiethylester .
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(diethoxymethylsilyl)ethyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (beta,gamma-epoxypropyl)phosphonate
- 1067-74-9
- 106778-42-1
- 106778-43-2
- 1067-87-4
- 1067883-56-0
- 1067-90-9
- 106792-29-4
- 106792-38-5
- 106-79-6
- 106797-53-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View