-
Name
GLYCOLALDEHYDE DIMETHYL ACETAL
- EINECS 250-398-7
- CAS No. 30934-97-5
- Article Data22
- CAS DataBase
- Density 1.009 g/cm3
- Solubility Miscible with water.
- Melting Point <-76°C
- Formula C4H10O3
- Boiling Point 146.1 °C at 760 mmHg
- Molecular Weight 106.122
- Flash Point 42.2 °C
- Transport Information
- Appearance
- Safety 23-24/25
- Risk Codes 23-24/25
-
Molecular Structure
- Hazard Symbols
- Synonyms Glycolaldehyde,dimethyl acetal (6CI,7CI);2,2-Dimethoxyethanol;Hydroxyacetaldehyde dimethylacetal;2,2-dimethoxyethanol;
- PSA 38.69000
- LogP -0.40240
Synthetic route
-
-
23147-58-2, 110822-84-9, 110822-85-0
glycolaldehyde dimer

-
-
67-56-1
methanol

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| aminopropylated Silica-Gel hydrochloride (APSG*HCl) resin for 15h; Ambient temperature; | 92% |
| With hydrogenchloride at 89.84℃; for 1h; Reagent/catalyst; Time; | 72 %Chromat. |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride for 3h; Irradiation; | 68% |
-
-
67-56-1
methanol

-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
91-09-8, 612-05-5, 1825-00-9, 2500-78-9, 3867-83-2, 3945-28-6, 5328-63-2, 6206-67-3, 6207-01-8, 6207-03-0, 7404-24-2, 17289-61-1, 18449-76-8, 33509-64-7, 36793-06-3, 41897-31-8, 41897-32-9, 41897-33-0, 41897-34-1, 41897-37-4, 41897-38-5, 41897-39-6, 53448-52-5, 65137-86-2, 89615-04-3, 89615-05-4, 131233-91-5, 14703-09-4
methyl xyloside

-
B
-
30934-97-5
2,2-dimethoxyethanol

-
C
-
547-64-8
methyl lactate

| Conditions | Yield |
|---|---|
| With Ti-beta zeolite at 160℃; for 2h; Microwave irradiation; | A 48% B 7% C 11% |
| With Zr-beta zeolite at 160℃; for 2h; Microwave irradiation; | A 39% B 12% C 10% |


| Conditions | Yield |
|---|---|
| With titanium tetrachloride for 3h; Irradiation; | 37% |
-
-
67-56-1
methanol

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 35% |
| With triethylamine; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 35% |
| With potassium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 20℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 27% |
| With caesium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 20℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 27% |
| With potassium hydroxide; dichloro(pentamethylcyclopentadienyl) iridium In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 20% |

-
-
67-56-1
methanol

-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
98-01-1
furfural

-
B
-
1453-62-9
2-furaldehyde dimethyl acetal

-
C
-
91-09-8, 612-05-5, 1825-00-9, 2500-78-9, 3867-83-2, 3945-28-6, 5328-63-2, 6206-67-3, 6207-01-8, 6207-03-0, 7404-24-2, 17289-61-1, 18449-76-8, 33509-64-7, 36793-06-3, 41897-31-8, 41897-32-9, 41897-33-0, 41897-34-1, 41897-37-4, 41897-38-5, 41897-39-6, 53448-52-5, 65137-86-2, 89615-04-3, 89615-05-4, 131233-91-5, 14703-09-4
methyl xyloside

-
D
-
30934-97-5
2,2-dimethoxyethanol

-
E
-
547-64-8
methyl lactate

| Conditions | Yield |
|---|---|
| With Sn-beta zeolite at 160℃; for 2h; Microwave irradiation; | A n/a B n/a C 32% D 6% E 11% F 12% |

-
-
67-56-1
methanol

-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
91-09-8, 612-05-5, 1825-00-9, 2500-78-9, 3867-83-2, 3945-28-6, 5328-63-2, 6206-67-3, 6207-01-8, 6207-03-0, 7404-24-2, 17289-61-1, 18449-76-8, 33509-64-7, 36793-06-3, 41897-31-8, 41897-32-9, 41897-33-0, 41897-34-1, 41897-37-4, 41897-38-5, 41897-39-6, 53448-52-5, 65137-86-2, 89615-04-3, 89615-05-4, 131233-91-5, 14703-09-4
methyl xyloside

-
B
-
30934-97-5
2,2-dimethoxyethanol

-
C
-
547-64-8
methyl lactate

| Conditions | Yield |
|---|---|
| With Sn-beta zeolite at 160℃; for 2h; Microwave irradiation; | A 30% B 6% C 17% D 11% |

-
-
127657-97-0
2-benzyloxy-1,1-dimethoxyethane

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With diethyl ether; ammonia; sodium |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With S2O82-/silicon MCM-41 at 100℃; for 3h; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid |

| Conditions | Yield |
|---|---|
| (i) Br2, Et2O, (ii) /BRN= 3592982/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate anodic oxidation; |
-
-
67-56-1
methanol

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With titanium tetrachloride for 15h; Irradiation; Yield given; |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid; sulfuric acid 1a) MeOH, 0 deg C, 1.5h, 1b) RT, overnight, 2) 1h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium hydrogencarbonate; acetonitrile; sulfuric acid 1a) MeOH, 0 deg C, 1.5h, 2) 12h, reflux, 2) MeOH, 1h, reflux; Yield given. Multistep reaction; |
-
-
67-56-1
methanol

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
96-35-5
glycolic acid methyl ester

| Conditions | Yield |
|---|---|
| dichloro(pentamethylcyclopentadienyl) iridium In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | |
| With potassium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | |
| With caesium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | |
| With potassium hydroxide; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | |
| With sodium hydroxide; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 120℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 5 %Chromat. B 31 %Chromat. |
-
-
58-86-6
D-xylose

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

-
C
-
5837-73-0
methyl 2-hydroxybut-3-enoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 160℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 5 %Chromat. B 42 %Chromat. C 7 %Chromat. |

-
-
10323-20-3
D-Arabinose

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

-
C
-
5837-73-0
methyl 2-hydroxybut-3-enoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 160℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 6 %Chromat. B 39 %Chromat. C 8 %Chromat. |

-
-
50-69-1
D-ribose

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

-
C
-
5837-73-0
methyl 2-hydroxybut-3-enoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 160℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 5 %Chromat. B 38 %Chromat. C 8 %Chromat. |

-
-
1114-34-7
D-lyxose

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

-
C
-
5837-73-0
methyl 2-hydroxybut-3-enoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 160℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 6 %Chromat. B 39 %Chromat. C 7 %Chromat. |

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

-
C
-
5837-73-0
methyl 2-hydroxybut-3-enoate

-
D
-
1361017-70-0
methyl 2-hydroxy-4-methoxybutanoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 120℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 7 %Chromat. B 10 %Chromat. C 25 %Chromat. D 19 %Chromat. |
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 140℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 8 %Chromat. B 14 %Chromat. C 30 %Chromat. D 12 %Chromat. |
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 160℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 7 %Chromat. B 16 %Chromat. C 27 %Chromat. D 6 %Chromat. |

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
5837-73-0
methyl 2-hydroxybut-3-enoate

-
C
-
1361017-70-0
methyl 2-hydroxy-4-methoxybutanoate

| Conditions | Yield |
|---|---|
| With zeotype catalyst Sn-Beta (Si/Sn = 400) In methanol at 100℃; under 15001.5 Torr; for 16h; Autoclave; Inert atmosphere; | A 8 %Chromat. B 13 %Chromat. C 26 %Chromat. |

-
-
23147-58-2, 110822-84-9, 110822-85-0
glycolaldehyde dimer

-
-
67-56-1
methanol

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
1361017-70-0
methyl 2-hydroxy-4-methoxybutanoate

| Conditions | Yield |
|---|---|
| With tin (IV) chloride pentahydrate at 89.84℃; for 1h; Kinetics; Reagent/catalyst; Time; | A 54 %Chromat. B 10 %Chromat. |

-
-
67-56-1
methanol

-
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
D-xylofuranose

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
547-64-8
methyl lactate

| Conditions | Yield |
|---|---|
| at 240℃; under 20686.5 Torr; for 1h; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| at 240℃; under 20686.5 Torr; for 1h; Inert atmosphere; |
-
-
67-56-1
methanol

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
547-64-8
methyl lactate

| Conditions | Yield |
|---|---|
| With mesoporous Zr-SBA-15 at 240℃; under 20686.5 Torr; for 1h; Inert atmosphere; |

-
-
89-91-8
Methyl dimethoxyacetate

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 3h; | 5.45 g |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 0 - 20℃; for 4h; | 100% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
79-30-1
isobutyryl chloride

-
-
791121-01-2
isobutyric acid 2,2-dimethoxy-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine at 20℃; for 16h; | 99% |
| With dmap; triethylamine In ethyl acetate at 0 - 20℃; for 16h; | 99% |
| With dmap; triethylamine In tert-butyl methyl ether at 0 - 20℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In DMF (N,N-dimethyl-formamide) at 0 - 20℃; | 93% |
| With sodium hydroxide In N,N-dimethyl-formamide at 0 - 20℃; for 16h; | 60% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; palladium diacetate; (S)-(-)-(6,6’-dimethoxybiphenyl-2,2’-diyl)bis(diphenylphosphine) In chlorobenzene; acetone at 20 - 80℃; for 30h; Schlenk technique; enantioselective reaction; | 93% |

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
1694-92-4
2-Nitrobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane at 20℃; for 3h; | 92% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 24h; Heating; | 91% |
| With hydrogenchloride In ethanol; water at -5℃; for 24h; Reflux; | 91% |
-
-
10486-61-0
3-thienyl iodide

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
1080649-34-8
3-(2,2-dimethoxyethoxy)thiophene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In toluene at 110℃; for 36h; | 91% |

| Conditions | Yield |
|---|---|
| With Montmorillonite K10 at 20℃; for 1.5h; | 88% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
153034-80-1
2-Fluoro-4-iodo-3-methylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethoxyethanol With sodium hydride In tetrahydrofuran Stage #2: 2-Fluoro-4-iodo-3-methylpyridine In tetrahydrofuran at 20℃; Further stages.; | 86% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 120h; | 85% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran -78 deg C -> r.t.; | 82% |
| With 2-chloro-1-methyl-pyridinium iodide; triethylamine In dichloromethane for 24h; Ambient temperature; | 81% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
2614-88-2
sorbinyl chloride

-
-
134856-14-7
2,2-dimethoxyethyl (2E,4E)-2,4-hexadienoate

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran for 9h; Ambient temperature; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethoxyethanol With sodium hydride In N,N-dimethyl-formamide for 0.5h; Stage #2: 2-Chloroquinoline In N,N-dimethyl-formamide at 20℃; for 24h; | 81% |
-
-
1810-72-6
2,6-dichloroquinoline

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethoxyethanol With sodium hydride In N,N-dimethyl-formamide for 0.5h; Stage #2: 2,6-dichloroquinoline In N,N-dimethyl-formamide at 20℃; for 24h; | 81% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
400835-49-6
ethyl-2-O-benzyl-6-O-isobutyl-3,4-di-O-methyl-1-thio-α-D-mannopyranoside

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 4 A molecular sieve; silver trifluoromethanesulfonate In dichloromethane; toluene at 0℃; | 80% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
647029-76-3
ethyl-2-O-benzyl-6-O-isopropyl-3,4-di-O-methyl-1-thio-α-D-mannopyranoside

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 4 A molecular sieve; silver trifluoromethanesulfonate In dichloromethane; toluene at 0℃; | 80% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
817160-11-5
N-(2-(((tert-butyldimethylsilyl)oxy)methyl)phenyl)-4-nitrobenzenesulfonamide

-
-
817160-13-7
N-[2-(tert-butyldimethylsilanyloxymethyl)phenyl]-N-(2,2-dimethoxyethyl)-4-nitrobenzenesulfonamide

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at -20 - 30℃; for 21h; Mitsunobu reaction; | 80% |
-
-
3350-78-5
3,3-Dimethylacryloyl chloride

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
134856-13-6
2,2-dimethoxyethyl 3-methyl-2-butenoate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; | 78% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
4224-69-5
methyl 2-(bromomethyl)propenoate

-
-
940279-21-0
2-(2,2-dimethoxy-ethoxymethyl)-acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0 - 20℃; | 78% |
-
-
120-72-9
indole

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
108-24-7
acetic anhydride

-
-
88321-08-8
2,2-di-(1H-indol-3-yl)ethyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: indole; 2,2-dimethoxyethanol at 20℃; for 1.5h; Stage #2: acetic anhydride With sodium acetate at 20℃; for 17h; | 76% |

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With silver carbonate In dichloromethane at 0℃; | 75% |
-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; silver carbonate In dichloromethane at 0℃; | 75% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
35661-39-3
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine

-
-
71989-33-8
Fmoc-Ser(tBu)-OH

-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
1426238-82-5
(3S,6S,8aS)-6-methyl-7-((4-nitrophenyl)sulfonyl)-5-oxohexahydro-2H-oxazolo[3,2-a]pyrazine-3-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-Ser(tBu)-OH With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 16h; Rink amide resin; Stage #2: With piperidine In N,N-dimethyl-formamide at 20℃; for 0.333333h; Rink amide resin; Stage #3: 2,2-dimethoxyethanol; N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine; 4-Nitrobenzenesulfonyl chloride stereoselective reaction; Further stages; | 75% |

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
1612171-78-4
6-chloro-2-(chloromethyl)-1-isopropyl-imidazo [4,5-c]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethoxyethanol With sodium hydride In N,N-dimethyl-formamide at 50℃; for 1h; Stage #2: 6-chloro-2-chloromethyl-1-isopropyl-1H-imidazo[4,5-c]pyridine In N,N-dimethyl-formamide at 50℃; for 2h; | 75% |
-
-
30934-97-5
2,2-dimethoxyethanol

-
-
113240-46-3
monoallyl malonate

-
-
68641-49-6
bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate | 73% |

-
-
30934-97-5
2,2-dimethoxyethanol

-
-
2046-18-6
4-phenylbutyronitrile

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 120h; | 73% |
-
-
2024-83-1
veratronitrile

-
-
30934-97-5
2,2-dimethoxyethanol

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In 1,2-dichloro-ethane at 20℃; for 24h; Concentration; Solvent; Temperature; | 68% |
Ethanol, 2,2-dimethoxy- Specification
The Ethanol, 2,2-dimethoxy-, with the CAS registry number 30934-97-5 and EINECS registry number 250-398-7, has the systematic name and IUPAC name of 2,2-dimethoxyethanol. And the molecular formula of the chemical is C4H10O3. What's more, while dealing with this chemical, you should avoid contacting with skin and eyes, and do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer).
The characteristics of Ethanol, 2,2-dimethoxy- are as followings: (1)ACD/LogP: -0.41; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.41; (4)ACD/LogD (pH 7.4): -0.41; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 14.31; (8)ACD/KOC (pH 7.4): 14.31; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 27.69 Å2; (13)Index of Refraction: 1.401; (14)Molar Refractivity: 25.56 cm3; (15)Molar Volume: 105.1 cm3; (16)Polarizability: 10.13×10-24cm3; (17)Surface Tension: 29.3 dyne/cm; (18)Density: 1.009 g/cm3; (19)Flash Point: 42.2 °C; (20)Enthalpy of Vaporization: 44.63 kJ/mol; (21)Boiling Point: 146.1 °C at 760 mmHg; (22)Vapour Pressure: 1.85 mmHg at 25°C.
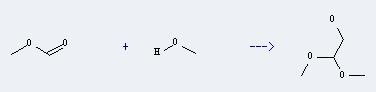
Preparation of Ethanol, 2,2-dimethoxy-: This chemical can be prepared by methanol and formic acid methyl ester. The reaction will need reagent titanium(IV) chloride. The reaction time is 3 hours with irridieation, and the yield is about 68%.
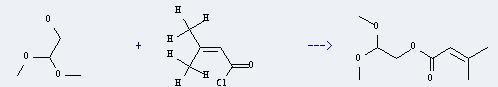
Uses of Ethanol, 2,2-dimethoxy-: It can react with 3-methyl-but-2-enoyl chloride to produce 2,2-dimethoxyethyl 3-methyl-2-butenoate. This reaction will need reagent pyridine, and the menstruum CH2Cl2. And the yield is about 78%.
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: OCC(OC)OC
(2)InChI: InChI=1/C4H10O3/c1-6-4(3-5)7-2/h4-5H,3H2,1-2H3
(3)InChIKey: NYPNCQTUZYWFGG-UHFFFAOYAP
Related Products
- Ethanol
- Ethanol, 2-(2,4,6-trichlorophenoxy)-
- Ethanol, 2-(2-pyridinylthio)-
- Ethanol, 2,2',2''-nitrilotri-, copper salt
- Ethanol, 2,2,2-nitrilotris-, compd. with alpha-(2,4,6-tris(1-phenylethyl)phenyl)-omega-hydroxypoly(oxy-1,2-ethanediyl) phosphate
- Ethanol, 2,2-difluoro-,1-acetate
- Ethanol, 2,2-dimethoxy-
- Ethanol, 2,2-dinitro-,potassium salt (1:1)
- Ethanol, 2,2'-sulfonylbis-
- Ethanol, 2-[(5-nitro-2-pyridinyl)oxy]-
- 309-36-4
- 3093-97-8
- 3094-09-5
- 309-43-3
- 30944-92-4
- 30947-30-9
- 30948-01-7
- 3094-87-9
- 30950-27-7
- 30951-66-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View