-
Name
Honokiol
- EINECS 609-119-8
- CAS No. 35354-74-6
- Article Data19
- CAS DataBase
- Density 1.107 g/cm3
- Solubility DMSO: 36 mg/mL
- Melting Point 87.5ºC
- Formula C18H18O2
- Boiling Point 400.1 °C at 760 mmHg
- Molecular Weight 266.34
- Flash Point 184 °C
- Transport Information UN 3077 9/PG 3
- Appearance Dark brown to white fine powder with pleasant odor, spicy and slightly bitter taste.
- Safety 26-39-61
- Risk Codes 41-51/53
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, N
N
- Synonyms [1,1'-Biphenyl]-2,4'-diol,3',5-di-2-propenyl- (9CI);3',5-Diallyl-2,4'-biphenyldiol;NSC 293100;Honokiol,(S);
- PSA 40.46000
- LogP 4.22180
Synthetic route
-
-
68592-18-7
Dimethylhonokiol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at -5 - 0℃; for 0.333333h; | 97% |
| With boron tribromide-dimethyl sulfide complex In 1,2-dichloro-ethane at 65℃; for 15h; Reagent/catalyst; Temperature; Inert atmosphere; | 95% |
| Stage #1: Dimethylhonokiol With triethylsilane; tris(pentafluorophenyl)borate In dichloromethane at 20℃; for 0.333333h; Inert atmosphere; Schlenk technique; Stage #2: With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Schlenk technique; Stage #3: With water In tetrahydrofuran | 57.8% |
-
-
68592-19-8
3,5′-diallyl-2′-methoxy-[1,1′-biphenyl]-4-ol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With boron trichloride - methyl sulfide complex In dichloromethane; 1,2-dichloro-ethane for 18h; Reflux; | 91% |
| With boron tribromide In dichloromethane at 20℃; for 0.416667h; Inert atmosphere; | 90% |
| With boron tribromide at -15 - 20℃; | |
| With boron tribromide In dichloromethane at 20℃; for 0.416667h; Inert atmosphere; | 430 mg |
-
-
108886-93-7
5-allyl-4'-(allyloxy)-[1,1'-biphenyl]-2-ol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With N,N-diethylaniline at 200℃; for 24h; Claisen Rearrangement; Sealed tube; Schlenk technique; Inert atmosphere; | 90% |
| With boron trichloride In chlorobenzene at -15℃; for 1h; | 80% |
-
-
711012-14-5
5,3'-diallyl-2,4'-bis-methoxymethoxy-biphenyl

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 20℃; for 44h; | 89% |

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With methanol; chloro-trimethyl-silane at 0 - 20℃; for 6h; | 85% |

| Conditions | Yield |
|---|---|
| for 1.33333h; Claisen Rearrangement; Microwave irradiation; Heating; Sealed tube; | A 40% B 60% |
| at 180℃; for 1.33333h; Claisen Rearrangement; Microwave irradiation; | A 40% B 60% |
| at 190℃; for 12h; Claisen Rearrangement; Sealed tube; | A 55% B 40% |
| at 190℃; for 12h; Claisen Rearrangement; Sealed tube; | A 55% B 40% |
-
-
1133087-04-3
5-allyl-4'-(allyloxy)-2-methoxybiphenyl

-
A
-
35354-74-6
Honokiol

-
B
-
1253950-18-3
5-allyl-[1,1'-biphenyl]-2,4'-diol

| Conditions | Yield |
|---|---|
| With boron trichloride - methyl sulfide complex In dichloromethane; 1,2-dichloro-ethane for 18h; Claisen rearrangement; Reflux; | A 47% B 49% |
-
-
126654-55-5
eudeshonokiol A

-
A
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene |
-
-
126654-55-5
eudeshonokiol A

-
A
-
35354-74-6
Honokiol

-
B
-
13902-07-3, 15051-81-7, 72173-98-9, 98683-12-6, 117066-77-0, 1209-71-8
eudesm-4-en-11-ol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene for 10h; Ambient temperature; | A 7 mg B 3 mg |
-
-
89-55-4
5-bromosalicyclic acid

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 100 percent / aq. H2SO4 / Heating 2: 100 percent / i-Pr2NEt / dimethylformamide / 3 h / 20 °C 3: 86 percent / PdCl2(dppf); dppf; AcOK / dioxane / 80 °C 4: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 5: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 6: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 7: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 8: 52 percent / CuI / tetrahydrofuran / -26 °C 9: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
29415-97-2
methyl 3-bromo-4-hydroxybenzoate

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 98 percent / i-Pr2NEt / dimethylformamide / 3 h / 20 °C 2: 100 percent / DIBAL-H / CH2Cl2 / 2 h / -78 °C 3: 100 percent / imidazole / dimethylformamide / 20 °C 4: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 5: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 6: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 7: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 8: 52 percent / CuI / tetrahydrofuran / -26 °C 9: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
4068-76-2
5-bromo-2-hydroxy-benzoic acid methyl ester

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 100 percent / i-Pr2NEt / dimethylformamide / 3 h / 20 °C 2: 86 percent / PdCl2(dppf); dppf; AcOK / dioxane / 80 °C 3: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 4: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 5: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 6: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 7: 52 percent / CuI / tetrahydrofuran / -26 °C 8: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
99-76-3
methyl 4-hydroxylbenzoate

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 80 percent / HBF4; N-bromosuccinimide / acetonitrile / -20 - 20 °C 2: 98 percent / i-Pr2NEt / dimethylformamide / 3 h / 20 °C 3: 100 percent / DIBAL-H / CH2Cl2 / 2 h / -78 °C 4: 100 percent / imidazole / dimethylformamide / 20 °C 5: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 6: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 7: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 8: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 9: 52 percent / CuI / tetrahydrofuran / -26 °C 10: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
162269-91-2
(3-bromo-4-methoxymethoxy-phenyl)-methanol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 100 percent / imidazole / dimethylformamide / 20 °C 2: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 3: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 4: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 5: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 6: 52 percent / CuI / tetrahydrofuran / -26 °C 7: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-12-3
5-bromo-2-methoxymethoxy-benzoic acid methyl ester

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 86 percent / PdCl2(dppf); dppf; AcOK / dioxane / 80 °C 2: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 3: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 4: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 5: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 6: 52 percent / CuI / tetrahydrofuran / -26 °C 7: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
672922-57-5
3-bromo-4-methoxymethoxybenzoic acid methyl ester

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 100 percent / DIBAL-H / CH2Cl2 / 2 h / -78 °C 2: 100 percent / imidazole / dimethylformamide / 20 °C 3: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 4: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 5: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 6: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 7: 52 percent / CuI / tetrahydrofuran / -26 °C 8: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-11-2
(3-bromo-4-methoxymethoxy-benzyloxy)-tert-butyl-dimethyl-silane

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 2: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 3: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 4: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 5: 52 percent / CuI / tetrahydrofuran / -26 °C 6: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 99 percent / aq. H2SO4 / Heating 2: 80 percent / HBF4; N-bromosuccinimide / acetonitrile / -20 - 20 °C 3: 98 percent / i-Pr2NEt / dimethylformamide / 3 h / 20 °C 4: 100 percent / DIBAL-H / CH2Cl2 / 2 h / -78 °C 5: 100 percent / imidazole / dimethylformamide / 20 °C 6: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 7: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 8: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 9: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 10: 52 percent / CuI / tetrahydrofuran / -26 °C 11: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-10-1
2-methoxymethoxy-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzoic acid methyl ester

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 87 percent / PdCl2(dppf); dppf; K3PO4 / dioxane / 108 °C 2: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 3: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 4: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 5: 52 percent / CuI / tetrahydrofuran / -26 °C 6: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-16-7
5,3'-bis-bromomethyl-2,4'-bis-methoxymethoxy-biphenyl

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / CuI / tetrahydrofuran / -26 °C 2: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-13-4
(3'-hydroxymethyl-6,4'-bis-methoxymethoxy-biphenyl-3-yl)-methanol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 2: 52 percent / CuI / tetrahydrofuran / -26 °C 3: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-09-8
5'-(tert-butyl-dimethyl-silanyloxymethyl)-4,2'-bis-methoxymethoxy-biphenyl-3-carboxylic acid methyl ester

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 99 percent / LiAlH4 / tetrahydrofuran / 0 °C 2: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 3: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 4: 52 percent / CuI / tetrahydrofuran / -26 °C 5: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
711012-15-6
[5'-(tert-butyl-dimethyl-silanyloxymethyl)-4,2'-bis-methoxymethoxy-biphenyl-3-yl]-methanol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 98 percent / HF; pyridine / acetonitrile / 1 h / 20 °C 2: 80 percent / N-bromosuccinimide; triphenylphosphine / CH2Cl2 3: 52 percent / CuI / tetrahydrofuran / -26 °C 4: 89 percent / aq. HCl / methanol / 44 h / 20 °C View Scheme |
-
-
501-92-8
4-(prop-2-enyl)phenol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 29.2 percent / acetic acid / 0.25 h / Ambient temperature 2: 1.) methyl iodide, Mg / 1.) ether, reflux, 5 h; 2.) ether, -15 deg C, 30 min 3: 80 percent / BCl3 / chlorobenzene / 1 h / -15 °C View Scheme |
-
-
108886-90-4
1-allyl-4-oxocyclohexa-2,5-dienylethanoate

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) methyl iodide, Mg / 1.) ether, reflux, 5 h; 2.) ether, -15 deg C, 30 min 2: 80 percent / BCl3 / chlorobenzene / 1 h / -15 °C View Scheme |
-
-
68592-15-4
methylhonokiol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Stage #1: methylhonokiol With boron tribromide In dichloromethane at -78℃; Stage #2: With water | |
| Grignard reaction; |
-
-
1133087-04-3
5-allyl-4'-(allyloxy)-2-methoxybiphenyl

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 2: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 2: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme |
-
-
87688-94-6
4-allyl-2-bromo-1-methoxybenzene

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: osmium(VIII) oxide; 4-methylmorpholine N-oxide / water; acetone; tert-butyl alcohol / 24 h / 20 °C / Inert atmosphere 2.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium carbonate / 1,2-dimethoxyethane; water / 18 h / 80 °C / Inert atmosphere 3.1: triethylamine / dichloromethane / 0.5 h / Inert atmosphere; Cooling with ice 3.2: 2.08 h / 20 °C / Inert atmosphere 3.3: 18 h / 140 °C / Inert atmosphere 4.1: sodium iodide; zinc / N,N-dimethyl-formamide / 18 h / 140 °C / Inert atmosphere 5.1: potassium carbonate / acetone / 20 °C / Inert atmosphere 6.1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 7.1: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: osmium(VIII) oxide; water; 4-methylmorpholine N-oxide / acetone; tert-butyl alcohol / 24 h / 20 °C / Inert atmosphere 2.1: sodium carbonate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,2-dimethoxyethane; water / 18 h / 80 °C / Inert atmosphere 3.1: triethylamine / dichloromethane / 0.5 h / Inert atmosphere; Cooling 3.2: 2 h / 20 °C / Inert atmosphere; Cooling 3.3: 18 h / 140 °C / Inert atmosphere 4.1: potassium carbonate / acetone / 20 °C / Inert atmosphere 5.1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 6.1: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme |
-
-
1133087-02-1
5'-allyl-2'-methoxy-biphenyl-4-ol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetone / 20 °C / Inert atmosphere 2: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 3: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / acetone / 20 °C / Inert atmosphere 2: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 3: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme |
-
-
1355483-89-4
3-(3-bromo-4-methoxy-phenyl)-propane-1,2-diol

-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium carbonate / 1,2-dimethoxyethane; water / 18 h / 80 °C / Inert atmosphere 2.1: triethylamine / dichloromethane / 0.5 h / Inert atmosphere; Cooling with ice 2.2: 2.08 h / 20 °C / Inert atmosphere 2.3: 18 h / 140 °C / Inert atmosphere 3.1: sodium iodide; zinc / N,N-dimethyl-formamide / 18 h / 140 °C / Inert atmosphere 4.1: potassium carbonate / acetone / 20 °C / Inert atmosphere 5.1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 6.1: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium carbonate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,2-dimethoxyethane; water / 18 h / 80 °C / Inert atmosphere 2.1: triethylamine / dichloromethane / 0.5 h / Inert atmosphere; Cooling 2.2: 2 h / 20 °C / Inert atmosphere; Cooling 2.3: 18 h / 140 °C / Inert atmosphere 3.1: potassium carbonate / acetone / 20 °C / Inert atmosphere 4.1: diethylaluminium chloride / hexane / 2 h / 20 °C / Inert atmosphere 5.1: boron tribromide / dichloromethane / 0.42 h / 20 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 1h; Cooling with ice; | 98.4% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 56℃; for 12h; | 97% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; toluene at 120℃; for 24h; | 95.5% |


| Conditions | Yield |
|---|---|
| In 1,4-dioxane; toluene at 110℃; for 43h; | 95.5% |


| Conditions | Yield |
|---|---|
| With pyridine | 95% |
| With potassium carbonate at 20℃; for 0.5h; | 56% |
| With pyridine at 25℃; for 18h; | |
| With sodium hydrogencarbonate In ethyl acetate for 48h; |
-
-
35354-74-6
Honokiol

-
-
35406-31-6
Tetrahydrohonokiol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol for 5h; Reflux; | 94% |
| With sodium tetrahydroborate; nickel dichloride | 70% |
| With hydrogen; palladium on activated charcoal | |
| With palladium on activated charcoal; hydrogen | |
| With Lindlar's catalyst; hydrogen In ethanol at 20℃; under 760.051 Torr; for 24h; |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; toluene at 100℃; for 58h; | 93.6% |


| Conditions | Yield |
|---|---|
| In 1,4-dioxane; toluene at 100℃; for 52h; | 90.8% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 40℃; for 48h; Inert atmosphere; | 90% |
-
-
35354-74-6
Honokiol

-
-
105-36-2
ethyl bromoacetate

-
-
1333391-01-7
diethyl 2,2'-((3',5-diallyl-[1,1'-biphenyl]-2,4'-diyl)bis(oxy))diacetate

| Conditions | Yield |
|---|---|
| Stage #1: Honokiol With potassium carbonate In N,N-dimethyl-formamide at 20 - 25℃; Williamson synthesis; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide at 30℃; for 6h; Williamson synthesis; | 89.3% |
-
-
35354-74-6
Honokiol

-
-
1416567-14-0
4-allyl-2-(2-methyl-benzofuran-5-yl)phenol

| Conditions | Yield |
|---|---|
| With oxygen; sodium acetate; palladium dichloride In N,N-dimethyl acetamide; water at 60℃; under 6080.41 Torr; for 16h; Autoclave; | 86% |
| With oxygen; sodium acetate; palladium dichloride In N,N-dimethyl acetamide; water at 60℃; under 6080.41 Torr; for 16h; Autoclave; | 86% |
| With oxygen; sodium acetate; palladium dichloride In N,N-dimethyl acetamide; water at 60℃; under 6000.6 Torr; for 16h; Wacker Oxidation; | 86% |

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 16h; | 86% |
| Stage #1: formaldehyd; dimethyl amine With hydrogenchloride In methanol; water at 2 - 35℃; for 1h; Stage #2: Honokiol In methanol; water at 75℃; for 6h; | 60% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 84% |
| With sodium hydride In water; dimethyl sulfoxide at 0℃; | 38% |
-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water; acetonitrile at 20℃; for 1.5h; Cooling with ice; | 79% |
| With hydrogenchloride; sodium nitrite In water; acetonitrile at 20℃; for 1.5h; | 79.3% |
-
-
35354-74-6
Honokiol

-
-
4392-24-9
3-bromo-1-phenyl-1-propenyl bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; | 78% |
-
-
35354-74-6
Honokiol

-
-
67-68-5
dimethyl sulfoxide

| Conditions | Yield |
|---|---|
| With ammonium iodide at 130℃; for 18h; Schlenk technique; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; dimethyl amine In water at 55℃; for 0.666667h; Mannich Aminomethylation; Stage #2: Honokiol In water | 76% |

-
-
35354-74-6
Honokiol

-
-
264882-00-0
methyl 2-(2-chlorophenyl)-2-diazoacetate

| Conditions | Yield |
|---|---|
| With sewage sludge-derived carbon material treated with perchloric acid In 1,2-dichloro-ethane at 70℃; for 24h; | 72% |

| Conditions | Yield |
|---|---|
| With sodium In ethanol for 5h; Inert atmosphere; Reflux; | 70% |
-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Stage #1: Honokiol With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 2h; Stage #2: With sodium hydroxide In tetrahydrofuran for 1h; Reflux; | 66% |
| Multi-step reaction with 2 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 20 °C 2: sodium hydroxide; water / tetrahydrofuran / 1 h / Reflux View Scheme |
-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| With iodine In ethanol; water at 50℃; for 12h; | 65.87% |
-
-
50-00-0
formaldehyd

-
-
35354-74-6
Honokiol

-
-
1333391-05-1
3',5-diallyl-3,5'-bis(hydroxymethyl)biphenyl-2,4'-diol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 24h; | 63.15% |
-
-
35354-74-6
Honokiol

| Conditions | Yield |
|---|---|
| Stage #1: Honokiol With acetic acid In dichloromethane for 0.0833333h; Cooling with ice; Stage #2: With nitric acid In dichloromethane at 0℃; for 6h; | 61.3% |
| With nitric acid; acetic acid In dichloromethane at 10 - 20℃; for 1h; | 52% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; potassium iodide In acetone at 20℃; for 24h; | 61% |
Honokiol Chemical Properties
Molecular Structure of Honokiol (CAS NO.35354-74-6):
.png)
Molecular Formula: C18H18O2
Molecular Weight: 266.3343
IUPAC Name: 2-(4-Hydroxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol
Synonyms of Honokiol (CAS NO.35354-74-6): NSC 293100 ; 3,5'-Diallyl-4,2'-dihydroxybiphenyl
CAS NO: 35354-74-6
Classification Code: Anti-allergic agents ; Anti-anxiety agents ; Anti-arrhythmia agents ; Anti-infective agents ; Antineoplastic Agents ; Antineoplastic agents, phytogenic ; Cardiovascular Agents ; Central Nervous System Agents ; Central nervous system depressants ; Enzyme Inhibitors ; Gastrointestinal agents ; Psychotropic Drugs ; Tranquilizing Agents
Polar Surface Area: 18.46 Å2
Index of Refraction: 1.601
Molar Refractivity: 82.42 cm3
Molar Volume: 240.4 cm3
Surface Tension: 44.6 dyne/cm
Density of Honokiol (CAS NO.35354-74-6): 1.107 g/cm3
Flash Point: 184 °C
Enthalpy of Vaporization: 67.63 kJ/mol
Boiling Point: 400.1 °C at 760 mmHg
Vapour Pressure: 5.65E-07 mmHg at 25°C
Honokiol History
In the late 1990s, honokiol(35354-74-6) saw a revival in western countries as a potent and highly tolerable anti-tumorigenic and neurotrophic compound.
Honokiol Uses
Honokiol (CAS NO.35354-74-6) has been used in the traditional Japanese medicine Saiboku-to as an anxiolytic, antithrombotic, anti-depressant, anti-emetic, and anti-bacterial. In the late 1990s, It saw a revival in western countries as a potent and highly tolerable anti-tumorigenic and neurotrophic compound.
Honokiol Production
Synthesis
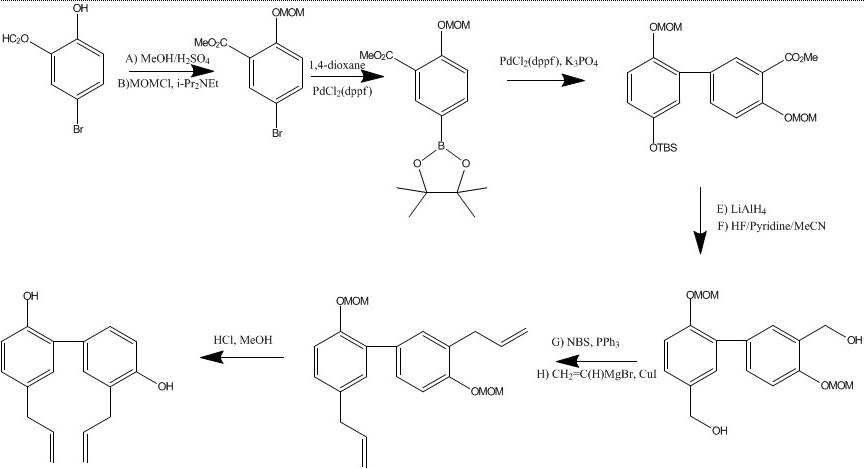
Honokiol Safety Profile
Hazard Codes of Honokiol (CAS NO.35354-74-6):  Xi,
Xi, N
N
Risk Statements: 41-51/53
R41: Risk of serious damage to the eyes.
R51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 26-39-61
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S39: Wear eye / face protection.
S61: Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 3077 9/PG 3
WGK Germany: 3
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View