 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water; dimethyl sulfoxide at 60℃; for 4.5h; High pressure; Green chemistry; | 99.9% |
| With borane-ammonia complex; copper cobaltite In methanol; water at 50℃; under 1125.11 Torr; for 5h; Catalytic behavior; Reagent/catalyst; Pressure; Concentration; Sealed tube; | 98% |
| With hydrogen In ethanol at 99.84℃; under 22502.3 Torr; Autoclave; | 81% |

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In methanol; water at 20℃; for 0.0833333h; | 99% |
| With ammonia borane; Pd/MIL-101 In methanol; water at 20℃; for 0.025h; | 99% |
| With sodium tetrahydroborate In methanol; water at 20℃; for 0.0666667h; Sealed tube; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 109.84℃; under 18751.9 Torr; for 7.5h; Autoclave; Green chemistry; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: propiononitrile In methanol; water at 20℃; for 0.0833333h; Stage #2: With ammonia borane In methanol; water at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen In para-xylene; isopropyl alcohol at 25℃; under 760.051 Torr; for 2h; Catalytic behavior; Reagent/catalyst; | A 6% B 94% |
| Pt-Rh catalysts Product distribution; electrochemical reduction; | |
| With sulfuric acid; palladium Product distribution; electrocatalytic hydrogenation, oth. catalysts; | |
| With 5% Pd/SiO2; hydrogen In isopropyl alcohol at 20℃; under 750.075 Torr; for 2h; | A 9 %Spectr. B 91 %Spectr. |
-
-
5300-22-1
(R/S)-2-(ethylamino)-1-phenylethanol

-
A
-
52500-61-5
2-hydroxy-1-phenylpropylamine

-
B
-
50-00-0
formaldehyd

-
C
-
100-52-7
benzaldehyde

-
D
-
75-04-7
ethylamine

-
E
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 1.9% B 67% C 75% D 79% E 10% |

-
-
3710-84-7
N-ethyl-N-hydroxy-ethanamine

-
A
-
50-00-0
formaldehyd

-
B
-
624-78-2
N-Ethylmethylamine

-
C
-
75-04-7
ethylamine

-
D
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In toluene at 18 - 20℃; Mechanism; Product distribution; object of study: Stieglitz rearrangement (prototype); | A n/a B 1.4% C 76% D n/a |

-
-
19528-69-9
{(C2H5NH2)2BH2}(1+)*{SC2H5}(1-)={(C2H5NH2)2BH2}{SC2H5}

-
A
-
7360-03-4
N,N',N''-triethylborazine

-
B
-
75-04-7
ethylamine

-
C
-
856627-30-0
ethylamine borane complex

-
D
-
75-08-1
ethanethiol

| Conditions | Yield |
|---|---|
| In neat (no solvent) pyrolysis;; | A 20% B n/a C 36% D 75% |
| In neat (no solvent) |

-
-
100-37-8
2-(Diethylamino)ethanol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
75-07-0
acetaldehyde

-
D
-
109-89-7
diethylamine

-
E
-
110-73-6
2-(Ethylamino)ethanol

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 5% B 1.6% C 72% D 20% E 73% |

-
-
623-76-7
N,N'-diethylurea

-
-
18522-92-4
sodium p-toluenesulfonamide

-
A
-
1467-23-8
N-(ethylcarbamoyl)-4-methylbenzenesulfonamide

-
B
-
75-04-7
ethylamine

| Conditions | Yield |
|---|---|
| at 145℃; for 5h; Product distribution; other time;; | A 71.6% B n/a |
| at 145℃; for 5h; | A 71.6% B n/a |

-
-
82922-12-1
2-Diethylamino-pentan-3-ol

-
A
-
75-04-7
ethylamine

-
B
-
75-07-0
acetaldehyde

-
C
-
123-38-6
propionaldehyde

-
-
100910-76-7
2-Ethylamino-pentan-3-ol

-
E
-
109-89-7
diethylamine

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A n/a B 70% C 36% D n/a E 46% |

-
-
82922-13-2
2-(ethylamino)-2-methylpropanol hydrochloride

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
124-68-5
2-Amino-2-methyl-1-propanol

-
D
-
75-07-0
acetaldehyde

-
E
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 50% B 44% C 14% D 26% E 66% |

-
-
82922-13-2
2-(ethylamino)-2-methylpropanol hydrochloride

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
124-68-5
2-Amino-2-methyl-1-propanol

-
D
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; anodic oxidation, pH 10, carbonate buffer; Further byproducts given; | A 50% B 44% C 14% D 66% |


| Conditions | Yield |
|---|---|
| With ammonium formate; zinc In methanol for 0.05h; Heating; | 62% |
| With ammonium amalgam | |
| With ammonium formate; magnesium In methanol at 20℃; for 0.666667h; |
-
-
2683-58-1
1-diethylamino-butan-2-ol

-
A
-
50-00-0
formaldehyd

-
B
-
68058-17-3
1-ethylamino-butan-2-ol

-
C
-
75-04-7
ethylamine

-
D
-
75-07-0
acetaldehyde

-
E
-
123-38-6
propionaldehyde

-
F
-
109-89-7
diethylamine

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 17% B 59% C 3% D 60% E 18% F 32% |


| Conditions | Yield |
|---|---|
| With K2CO3; THF In tetrahydrofuran Irradiation (UV/VIS); Et2NNO and K2CO3 added to soln. of Mo complex in THF, heated under reflux under N2 with W-light irrdn. for 4 h; filtered hot, cooled to room temp., crystals recrystd. from THF; elem. anal.; | A n/a B 60% C n/a D n/a |

-
-
38451-07-9
1,6-diethyl-2,5-dithiobiurea

-
A
-
75-04-7
ethylamine

-
B
-
13625-52-0
4-ethyl-[1,2,4]triazolidine-3,5-dithione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | A n/a B 56% |
-
-
86465-00-1
1-Ethoxy-3-ethylamino-propan-2-ol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With chlorine dioxide In water for 1h; Product distribution; Ambient temperature; | A 4% B 55% C 53% |

-
-
86464-98-4
1-Cyclohexyloxy-3-ethylamino-propan-2-ol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
75-07-0
acetaldehyde

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With chlorine dioxide In water for 1h; Product distribution; Ambient temperature; | A 5% B 54% C 55% D 18% |

-
-
58461-93-1
1-ethylamino-3-phenoxy-propan-2-ol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
75-07-0
acetaldehyde

-
D
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With chlorine dioxide In water for 1h; Product distribution; Ambient temperature; | A 3% B 49% C 50% D 30% |

-
-
64-17-5
ethanol

-
-
112-53-8
1-dodecyl alcohol

-
-
75-05-8
acetonitrile

-
A
-
112-40-3
dodecane

-
B
-
74-84-0
ethane

-
C
-
35902-57-9
N-n-dodecyl-ethylamine

-
D
-
4271-27-6
diethyllaurylamine

-
E
-
75-04-7
ethylamine

-
F
-
121-44-8
triethylamine

| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; copper at 240℃; under 7600 Torr; Product distribution; other catalyst and composition; | A n/a B n/a C 15% D 30% E n/a F 50% |

-
-
110-73-6
2-(Ethylamino)ethanol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
141-43-5
ethanolamine

-
D
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With water at 25℃; anodic oxidation, pH 10, carbonate buffer; Further byproducts given; | A 14% B 43% C 45% D 44% |

-
-
110-73-6
2-(Ethylamino)ethanol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
141-43-5
ethanolamine

-
D
-
75-07-0
acetaldehyde

-
E
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 14% B 43% C 45% D 44% E n/a |

-
-
125908-31-8
1-ethyl-6-tolyl-2,5-dithiobiurea

-
A
-
14731-25-0
2-(4'-methylphenylamino)-Δ2-1,3,4-thiadiazoline-5-thione

-
B
-
106-49-0
p-toluidine

-
C
-
75-04-7
ethylamine

-
D
-
13625-52-0
4-ethyl-[1,2,4]triazolidine-3,5-dithione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | A 44% B n/a C n/a D 31% |

-
-
73825-96-4
1-(ethylamino)-2-methylpropan-2-ol

-
A
-
75-04-7
ethylamine

-
B
-
2854-16-2
1-Amino-2-methyl-propan-2-ol

-
C
-
75-07-0
acetaldehyde

-
D
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; anodic oxidation, pH 10, carbonate buffer; Further byproducts given; | A 35% B 18% C 34% D 43% |

-
-
73825-96-4
1-(ethylamino)-2-methylpropan-2-ol

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
2854-16-2
1-Amino-2-methyl-propan-2-ol

-
D
-
75-07-0
acetaldehyde

-
E
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 18% B 35% C 18% D 34% E 43% |

-
-
125908-34-1
1-ethyl-6-(4'-ethoxyphenyl)-2,5-dithiobiurea

-
A
-
68161-60-4
2-(4'-ethoxyphenylamino)-Δ2-1,3,4-thiadiazoline-5-thione

-
B
-
75-04-7
ethylamine

-
C
-
156-43-4
4-Ethoxyaniline

-
D
-
13625-52-0
4-ethyl-[1,2,4]triazolidine-3,5-dithione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | A 41% B n/a C n/a D 32% |

-
-
125908-33-0
1-ethyl-6-(4'-methoxyphenyl)-2,5-dithiobiurea

-
A
-
37844-24-9
2-(4'-methoxyphenylamino)-Δ2-1,3,4-thiadiazoline-5-thione

-
B
-
104-94-9
4-methoxy-aniline

-
C
-
75-04-7
ethylamine

-
D
-
13625-52-0
4-ethyl-[1,2,4]triazolidine-3,5-dithione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | A 40% B n/a C n/a D 34% |

-
-
61784-88-1
1-ethyl-6-phenyl-2,5-dithiobiurea

-
A
-
75-04-7
ethylamine

-
B
-
62-53-3
aniline

-
C
-
10253-83-5
5-phenylamino-3H-[1,3,4]thiadiazole-2-thione

-
D
-
13625-52-0
4-ethyl-[1,2,4]triazolidine-3,5-dithione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | A n/a B n/a C 38% D 33% |


| Conditions | Yield |
|---|---|
| at 20℃; under 600.048 - 750.06 Torr; Addition; soid-gas reaction; ring cleavage; | 100% |
| With water |

| Conditions | Yield |
|---|---|
| at 20℃; under 600.048 - 750.06 Torr; Addition; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; under 750.06 Torr; Solid phase reaction; gas-solid reaction; | 100% |
| With water | |
| In ethanol at 50℃; | |
| In tetrahydrofuran; ethanol at 20℃; for 12h; Inert atmosphere; | |
| In ethanol at 20℃; for 12h; |
-
-
3460-18-2
1,4-dibromo-2-nitrobenzene

-
-
75-04-7
ethylamine

-
-
56136-82-4
(4-bromo-2-nitrophenyl)ethylamine

| Conditions | Yield |
|---|---|
| In water at 100℃; for 24h; Sealed vessel; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane; water at 24.84℃; for 4h; | 100% |
| In diethyl ether for 2h; | 74% |

| Conditions | Yield |
|---|---|
| 100% |
-
-
99735-46-3
methyl (3S)-1-<(R)-1-phenylethyl>-5-oxo-3-pyrrolidinecarboxylate

-
-
75-04-7
ethylamine

| Conditions | Yield |
|---|---|
| In water at -10℃; for 1h; | 100% |
-
-
115975-33-2
N,N-Dimethyl-2,4-bis(trifluoroacetyl)-1-naphthylamine

-
-
75-04-7
ethylamine

-
-
115975-37-6
1-[1-Ethylamino-4-(2,2,2-trifluoro-acetyl)-naphthalen-2-yl]-2,2,2-trifluoro-ethanone

| Conditions | Yield |
|---|---|
| In water; acetonitrile for 2h; Ambient temperature; | 100% |
-
-
75-04-7
ethylamine

-
-
84054-96-6
ethyl 4-formyl-2-hydroxybenzoate

-
-
84054-99-9
4-[(Z)-Ethyliminomethyl]-2-hydroxy-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| for 0.333333h; | 100% |
-
-
75-04-7
ethylamine

-
-
100890-40-2
1-Methyl-6-methylsulfanyl-2,4-dioxo-3-phenyl-1,2,3,4-tetrahydro-pyrimidine-5-carbonitrile

-
-
105736-29-6
6-Ethylamino-1-methyl-2,4-dioxo-3-phenyl-1,2,3,4-tetrahydro-pyrimidine-5-carbonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile for 0.0833333h; Ambient temperature; | 100% |
-
-
75-04-7
ethylamine


| Conditions | Yield |
|---|---|
| In diethyl ether Heating; | 100% |
-
-
75-04-7
ethylamine


| Conditions | Yield |
|---|---|
| In diethyl ether Heating; | 100% |
-
-
75-04-7
ethylamine


| Conditions | Yield |
|---|---|
| In diethyl ether Heating; | 100% |
-
-
75-04-7
ethylamine

-
-
22113-86-6
ethylammonium nitrate

| Conditions | Yield |
|---|---|
| With nitric acid In water at 10℃; pH=7.3; | 100% |
| With nitric acid In water for 1h; pH=7.3; Cooling with ice; | 100% |
| With acide nitrique at 0℃; |
-
-
27210-68-0
1-diethylamino-2,4-dinitronaphthalene

-
-
75-04-7
ethylamine

-
A
-
27210-67-9
N-ethyl-2,4-dinitro-1-naphthylamine

-
B
-
109-89-7
diethylamine

| Conditions | Yield |
|---|---|
| In water at 30℃; for 1h; | A 100% B n/a |

-
-
75-04-7
ethylamine

-
-
168697-54-9
tetraethyl ethylenebisbenzoyloxymalonate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 72h; | 100% |
-
-
23596-82-9
3,4-bis(diphenylmethylene)-1,2-cyclobutanedione

-
-
75-04-7
ethylamine

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 0.5h; Ambient temperature; | 100% |
-
-
75-04-7
ethylamine

-
-
221636-53-9
N,N-dimethyl-5,7-bis(trifluoroacetyl)-8-quinolylamine

| Conditions | Yield |
|---|---|
| With water In acetonitrile for 1h; Ambient temperature; | 100% |
| With water In acetonitrile at 20℃; for 1h; Substitution; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; for 22h; | 100% |
| In tetrahydrofuran at 20℃; Acylation; | |
| With ammonia In methanol at 20℃; |

| Conditions | Yield |
|---|---|
| at 80℃; under 600.048 - 750.06 Torr; Addition; soid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; under 600.048 - 750.06 Torr; Addition; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; under 600.048 - 750.06 Torr; Substitution; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| under 750.06 Torr; Condensation; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 0℃; under 150.012 Torr; Addition; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 0℃; under 187.515 Torr; Solid phase reaction; gas-solid reaction; | 100% |
| With calcium oxide In ethanol; water at 20℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| at 20℃; under 600.048 Torr; for 24h; Addition; solid-gas reaction; ring cleavage; | 100% |

| Conditions | Yield |
|---|---|
| at 0℃; under 375.03 Torr; Addition; solid-gas reaction; | 100% |
-
-
75-04-7
ethylamine

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
59488-00-5
4-[(ethylimino)methyl]-N,N-dimethylbenzeneamine

| Conditions | Yield |
|---|---|
| at 20℃; under 750.06 Torr; Solid phase reaction; gas-solid reaction; | 100% |
-
-
75-04-7
ethylamine

-
-
551-06-4
1-isothiocyanatonaphthalene

-
-
2742-61-2
1,1-dimethyl-3-naphthalene-1-yl-thiourea

| Conditions | Yield |
|---|---|
| at 20℃; under 750.06 Torr; Addition; solid-gas reaction; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; under 750.06 Torr; Solid phase reaction; gas-solid reaction; | 100% |
| In methanol for 9h; Reflux; | 84% |
Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View



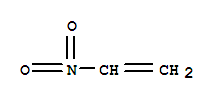












 F+,
F+, Xi,
Xi, T,
T, F
F