-
Name
Hydroxyprogesterone
- EINECS 200-699-4
- CAS No. 68-96-2
- Article Data86
- CAS DataBase
- Density 1.15 g/cm3
- Solubility 5.056mg/L(20 oC)
- Melting Point 276 °C
- Formula C21H20O3
- Boiling Point 482.9 °C at 760 mmHg
- Molecular Weight 330.467
- Flash Point 259.9 °C
- Transport Information
- Appearance White solid
- Safety 53-22-36/37/39-45
- Risk Codes 61
-
Molecular Structure
-
Hazard Symbols

- Synonyms 17-Hydroxypregn-4-ene-3,20-dione;17-Hydroxyprogesterone;17a-Hydroxylutin;17a-Hydroxypregn-4-ene-3,20-dione;17a-Hydroxyprogesterone;Gestageno;Gestageno Gador;Pregn-4-en-17a-ol-3,20-dione;Prodix;D4-Pregnen-17a-ol-3,20-dione;
- PSA 54.37000
- LogP 3.69430
Synthetic route
-
-
20380-16-9
17α-hydroxy-21-chloro-4-pregnene-3,20-dione

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With acetic acid; zinc | 100% |
| With titanium(III) sulphate; sulfuric acid; ammonia In methanol for 10h; | 83% |
-
-
1202062-99-4
cyclic-3-(1,2-ethanediylmercapto)-17α-hydroxypregn-4-en-20-one

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With water; calcium carbonate; methyl iodide In ethanol for 20h; Heating; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With mercaptoacetic acid In formamide for 0.25h; Ambient temperature; | 97.7% |

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 40℃; for 4h; pH=1.5 - 2; Temperature; Reagent/catalyst; | 97.4% |
-
-
83509-38-0
17β-Ethynyl-17α-hydroxyandrost-4-en-3-one

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With sulfuric acid; mercury(II) sulfate In water; acetone at 50 - 55℃; for 6h; Temperature; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: C22H31NO3; dimethyl zinc(II) With lithium chloride In toluene at 60 - 65℃; Stage #2: With hydrogenchloride In water at 55 - 60℃; Solvent; Reagent/catalyst; | 85.2% |

| Conditions | Yield |
|---|---|
| Stage #1: 16-dehydroprogesterone With phenylsilane; oxygen; isopropyl alcohol; Mn(dpm)3 In 1,2-dichloro-ethane at 0 - 25℃; Stage #2: With triethyl phosphite In 1,2-dichloro-ethane | 85% |
| Multi-step reaction with 2 steps 1: 79 percent / RhCl(PPh3)3 2: mCPBA / RhCl(PPh3)3 / CH2Cl2 View Scheme |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol | 85% |

-
-
4134-58-1, 17308-02-0, 41460-93-9, 41460-97-3, 630-56-8, 302-23-8
hydroxyprogesterone acetate

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol for 1h; Reflux; | 83.3% |
| With potassium hydroxide |
-
-
4470-79-5
21-Iodo-17α-hydroxypregn-4-en-3,20-dione

-
-
68-11-1
mercaptoacetic acid

-
A
-
68-96-2
17-hydroxyprogesterone

-
B
-
114967-85-0
(17α-Hydroxy-4-pregnene-3,20-dion-21-yl-21-thio)acetic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; | A 71.8% B 24.6% |

-
-
51154-62-2
(8S,9S,10R,13S,14S,Z)-17-ethylidene-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one

-
A
-
63-05-8
Androstenedione

-
B
-
68-96-2
17-hydroxyprogesterone

-
C
-
1662-06-2
17alpha,20beta-Dihydroxypregn-4-en-3-one

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; Oxone; sodium hydrogencarbonate In water; ethyl acetate; acetonitrile at -20 - 30℃; | A 10% B 70% C 20% |


| Conditions | Yield |
|---|---|
| With ruthenium trichloride; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; water at 20℃; | A 10% B 70% C 20% |


| Conditions | Yield |
|---|---|
| With tetrapropylammonium perruthennate; 4 A molecular sieve; 4-methylmorpholine N-oxide In dichloromethane; acetonitrile for 0.75h; | A 60% B 20% |
-
-
57-83-0
Progesterone

-
A
-
63-05-8
Androstenedione

-
B
-
68-96-2
17-hydroxyprogesterone

-
C
-
2243-08-5
6-oxoprogesterone

-
D
-
100979-81-5
A-nor-5β-pregnanedione-(3.20)

-
E
-
135574-05-9
4α-formyl-A-nor-pregnene-3,20-dione

-
F
-
55203-20-8
pregn-4-ene-3,12,20-trione

| Conditions | Yield |
|---|---|
| With 2-Picolinic acid; dihydrogen peroxide; iron(III) chloride In pyridine; acetic acid for 18h; Product distribution; Mechanism; Ambient temperature; | A 4.3% B 5.7% C 10.7% D 5% E 19.3% F 17.1% |

-
-
57-83-0
Progesterone

-
A
-
68-96-2
17-hydroxyprogesterone

-
B
-
2243-08-5
6-oxoprogesterone

-
C
-
135574-05-9
4α-formyl-A-nor-pregnene-3,20-dione

-
D
-
55203-20-8
pregn-4-ene-3,12,20-trione

| Conditions | Yield |
|---|---|
| With 2-Picolinic acid; dihydrogen peroxide; iron(III) chloride In pyridine; acetic acid for 18h; Ambient temperature; Further byproducts given; | A 5.7% B 10.7% C 19.3% D 17.1% |

-
-
145-13-1
Pregnenolone

-
A
-
58-22-0
testosterone

-
B
-
63-05-8
Androstenedione

-
C
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With glucose-6-phosphate dehydrogenase; propylene glycol; D-glucose; cortical tissue of mare adrenals at 37℃; for 2h; Product distribution; <3H>labelled; | A 0.15% B 5.1% C n/a |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
123-91-1
1,4-dioxane

-
-
60-29-7
diethyl ether

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| und Chromatographieren des Reaktionsprodukts an Aluminiumoxyd; |


| Conditions | Yield |
|---|---|
| at 110 - 130℃; |

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With sporormia minima | |
| With trichiderma viride |

| Conditions | Yield |
|---|---|
| With flavobacterium 'androstendionicum' |
-
-
83914-31-2
5α-Chloro-3β,17α-dihydroxypregnan-20-one

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With pyridine; chromium(VI) oxide anschliessend mit wss.NaOH; |
-
-
28439-56-7
5,6β-dichloro-3β,17-dihydroxy-5α-pregnan-20-one

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; chromium dichloride; sulfuric acid beim aufeinanderflogenden Behandeln; |
-
-
100733-64-0
17-hydroxy-16β-iodo-pregn-4-ene-3,20-dione

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With nickel |
-
-
418768-63-5
17-hydroxy-2ξ-iodo-pregn-4-ene-3,20-dione

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With chromium dichloride |
-
-
101978-51-2
3β,20βF-diacetoxy-pregnen-(5)-ol-(17)

-
-
108-94-1
cyclohexanone

-
-
555-31-7
aluminum isopropoxide

-
-
108-88-3
toluene

-
-
68-96-2
17-hydroxyprogesterone

-
-
13714-60-8
20,20-ethanediyldioxy-pregn-5-ene-3β,17-diol

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With cyclohexanone; aluminum isopropoxide anschliessend Behandeln mitwss.-methanol.H2SO4; |
-
-
67-56-1
methanol

-
-
623-11-0
1-methyl-4-nitrosobenzene

-
-
102813-01-4
20ξ-acetylamino-17,20ξ-epoxy-pregn-4-en-3-one

-
-
68-96-2
17-hydroxyprogesterone

-
-
102813-01-4
20ξ-acetylamino-17,20ξ-epoxy-pregn-4-en-3-one

-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
57-83-0
Progesterone

-
A
-
50-23-7
HYDROCORTISONE

-
B
-
68-96-2
17-hydroxyprogesterone

-
C
-
64-85-7
21-Hydroxyprogesterone

-
D
-
50-22-6
Corticosterone

-
E
-
152-58-9
Cortexolone

| Conditions | Yield |
|---|---|
| With glucose-6-phosphate dehydrogenase; phosphate buffer; air; α-D-glucose 6-phosphate; midterm female fetal human adrenal tissue; NADP; magnesium chloride at 37℃; for 2h; Product distribution; <3H>labeled; | A 36.3 % Chromat. B n/a C n/a D 4.7 % Chromat. E n/a |


| Conditions | Yield |
|---|---|
| With iodine; calcium chloride; calcium oxide In methanol; dichloromethane; water 1.) 10-12 deg C above room temp., 15 min., 2.) room temp., 30 min.; | 99.3% |
| With 2,2'-azobis(isobutyronitrile); iodine; calcium oxide In tetrahydrofuran; methanol at 20℃; for 5h; Inert atmosphere; | 90% |
| With tetrahydrofuran; iodine |

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 20℃; Electrochemical reaction; | 99% |
| With tetrapropylammonium perruthennate; 4 A molecular sieve; 4-methylmorpholine N-oxide In dichloromethane; acetonitrile for 0.75h; | 95% |
| With chromium(VI) oxide; acetic acid |
-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| Stage #1: 17-hydroxyprogesterone With pyrrolidine In methanol at 0℃; Reflux; Inert atmosphere; Stage #2: With hydrogenchloride; bromine In ethanol; water at 20℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 17-hydroxyprogesterone With calcium chloride; calcium oxide In methanol; chloroform for 2h; Stage #2: acetic anhydride With potassium carbonate; acetic acid In methanol; chloroform; N,N-dimethyl-formamide at 100℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 17-hydroxyprogesterone With pyrrolidine In ethanol at 0 - 50℃; Stage #2: potassium acetate With acetic acid; potassium iodide In ethanol; acetone at 20 - 60℃; | 98% |
-
-
68-96-2
17-hydroxyprogesterone

-
-
75863-28-4
m-Iodbenzoesaeureanhydrid

-
-
74176-91-3
17α-(m-iodobenzoyloxy)progesterone

| Conditions | Yield |
|---|---|
| With dmap In toluene at 50℃; for 18h; | 95% |
-
-
68-96-2
17-hydroxyprogesterone

-
-
110673-94-4
(8R,9S,10S,13S,14S,17R)-17-Acetyl-2,17-dihydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium tert-butylate; oxygen In toluene at -25℃; for 2.5h; | 95% |
-
-
68-96-2
17-hydroxyprogesterone

-
A
-
138430-10-1
(1R,8S,9S,10R,13S,14S,17R)-17-Acetyl-1,17-dihydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-2-oxa-cyclopenta[a]phenanthren-3-one

-
B
-
110673-96-6
(1S,8S,9S,10R,13S,14S,17R)-17-Acetyl-1,17-dihydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-2-oxa-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With crown ether; potassium tert-butylate; oxygen In toluene 1.) -25 deg C, 1.5-4 h, 2.) -20 deg C, 7 d; Title compound not separated from byproducts; | A n/a B 95% |
-
-
68-96-2
17-hydroxyprogesterone

-
-
108-24-7
acetic anhydride

-
-
4954-07-8
pregna-3,5-dien-20-one, 3,17-dihydroxy-, diacetate

| Conditions | Yield |
|---|---|
| With perchloric acid In ethyl acetate for 0.1h; | 92% |
| With perchloric acid In ethyl acetate at 20℃; for 0.5h; | 39% |
| With toluene-4-sulfonic acid | |
| With sodium hydrogen sulfate In tetrahydrofuran at 50℃; for 5h; Reagent/catalyst; Temperature; Solvent; |
-
-
68-96-2
17-hydroxyprogesterone

-
-
14510-23-7
17aβ-hydroxy-17aα-methyl-D-homoandrost-4-en-3,17-dione

| Conditions | Yield |
|---|---|
| iodine; zinc In benzene Heating; | 90% |
-
-
68-96-2
17-hydroxyprogesterone

-
-
66212-25-7
1,4,6-triene-3,20-dione-17α-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| Stage #1: 17-hydroxyprogesterone With pyridinium p-toluenesulfonate; orthoformic acid triethyl ester In ethanol at 39℃; for 6.5h; Stage #2: With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 87 - 90℃; for 3.5h; Temperature; Inert atmosphere; | 82.7% |

| Conditions | Yield |
|---|---|
| With pyridine; palladium 10% on activated carbon; hydrogen In 1,4-dioxane; ethanol at 30℃; for 17h; | 77% |
-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydroxide In methanol; dichloromethane; water at 20℃; for 24h; | 73% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0 - 5℃; for 1.5h; | A 28% B 70% |

| Conditions | Yield |
|---|---|
| Stage #1: 17-hydroxyprogesterone With C19H26ClIrN3O(1+)*Cl(1-) In water; acetonitrile at 80℃; for 0.166667h; Green chemistry; Stage #2: With formic acid In water; acetonitrile at 80℃; for 8h; Green chemistry; regioselective reaction; | 70% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 70% |
-
-
68-96-2
17-hydroxyprogesterone

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 24h; | A 11% B 66% |
Hydroxyprogesterone Chemical Properties
Product Name: Hydroxyprogesterone (CAS NO.68-96-2)
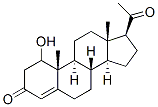
Molecular Formula: C21H30O3
Molecular Weight: 330.46g/mol
EINECS: 200-699-4
Appearance: White Solid
Melting Point: 276°C
Boiling point: 482.9 °C at 760 mmHg
Flash Point: 259.9 °C
Density: 1.15 g/cm3
Refractive index: 90 ° (C=1, CHCl3)
Surface Tension: 46.5 dyne/cm
Enthalpy of Vaporization: 86.18 kJ/mol
Vapour Pressure: 2.37E-11 mmHg at 25°C
XLogP3-AA: 3.2
H-Bond Donor: 1
H-Bond Acceptor: 3
Structure Descriptors of Hydroxyprogesterone (CAS NO.68-96-2):
IUPAC Name: (8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
Canonical SMILES: CC(=O)C1(CCC2C1(CCC3C2CCC4=CC(=O)CCC34C)C)O
Isomeric SMILES: CC(=O)[C@]1(CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C)O
InChI: InChI=1S/C21H30O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18,24H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1
InChIKey: DBPWSSGDRRHUNT-CEGNMAFCSA-N
Product Categories: Steroids; Biochemistry; Hydroxyketosteroids; Intermediates & Fine Chemicals; Pharmaceuticals
Hydroxyprogesterone Safety Profile
Safety Information of Hydroxyprogesterone (CAS NO.68-96-2):
Hazard Codes: T
Risk Statements: 61
R61:May cause harm to the unborn child.
Safety Statements: 53-22-36/37/39-45
S52:Not recommended for interior use on large surface areas.
S22:Do not breathe dust.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
WGK Germany: 3
RTECS: TU5060000
Hazardous Substances Data: 68-96-2(Hazardous Substances Data)
Hydroxyprogesterone Specification
Hydroxyprogesterone , its CAS NO. is 68-96-2, the synonyms are 17-Hydroxypregn-4-ene-3,20-dione ; 17-Hydroxyprogesterone ; Gestageno gador ; HSDB 3343 ; Hydroxyprogesteronum ; Idrossiprogesterone ; NSC 15468 ; Oxiprogesteronum ; Pregn-4-ene-3,20-dione, 17-hydroxy- ; Proluton ; Setaderm ; alpha Hydroxy progesterone ; delta(4)-Pregnene-17alpha-ol-3,20-dione .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View