-
Name
Ironpentacarbonyl
- EINECS 236-670-8
- CAS No. 13463-40-6
- Article Data264
- CAS DataBase
- Density 1.49 g/mL at 25 °C(lit.)
- Solubility Solubility in organic solvents
- Melting Point -20 °C
- Formula C5Fe O5
- Boiling Point 103 °C(lit.)
- Molecular Weight 195.899
- Flash Point 5 °F
- Transport Information UN 1994 6
- Appearance straw-yellow liquid
-
Safety
A poison by inhalation, skin contact, ingestion, subcutaneous, and intravenous routes. Inhalation causes dizziness, nausea, and vomiting. If continued, unconsciousness follows. Often there is a delayed reaction of chest pain, cough, and difficult breathing. There may be cyanosis and circulatory collapse. In fatal cases, death occurs from the fourth to eleventh day with pneumonitis and injury to kidneys, liver, and brain. Iron carbonyl is less toxic than nickel carbonyl.
A very dangerous fire and moderate explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Warning: pyrophoric in air. Mixtures with nitrogen oxide explode above 50°C. Violent reaction with zinc + transition metal halides (e.g., cobalt halides, rhodium halides, ruthenium halides). Mixtures with acetic acid + water produce a pyrophoric powder. To fight fire, use water, foam, CO2, dry chemical. See also CARBONYLS and IRON COMPOUNDS.
- Risk Codes 11-24-26/28
-
Molecular Structure
- Hazard Symbols Flammable, dangerous fire risk. Toxic by ingestion, inhalation, and skin absorption. TLV: TWA 0.1 ppm (Fe); STEL 0.2 ppm.
- Synonyms Ironcarbonyl (Fe(CO)5) (8CI); Iron pentacarbonyl; Iron pentacarbonyl (Fe(CO)5);Pentacarbonyl iron; R 20
- PSA 0.00000
- LogP -1.04250
Synthetic route
-
-
15321-51-4
diiron nonacarbonyl

-
-
103932-33-8
Os{PCO(CF3)}(CO)2(P(C6H5)3)2

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In tetrahydrofuran educts suspended in THF; after 1 h solvent removed in vacuo;; residue left under vacuum for 1 h; dissolved in CH2Cl2; addn. of ethanol; concentration; filtration; washed with ethanol and n-hexane; elem. anal.; | A n/a B 100% |

-
-
17685-52-8
triiron dodecarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| With carbon monoxide In n-heptane Irradiation (UV/VIS); soln. of Fe3(CO)12 in n-heptane is irradiated for 30 min in slow streamof CO; | 98% |
| In n-heptane Irradiation (UV/VIS); soln. of Fe3(CO)12 in n-heptane is irradiated at 293 K for 75 min in slow stream N2; | 96% |
| With carbon monoxide In N,N-dimethyl-formamide decompn. in presence of CO (1 atm) betwwen 0 and 25°C; intermediates observed;; not isolated; detn. by ESR; |
-
-
15321-51-4
diiron nonacarbonyl

-
-
124686-70-0
((t-Bu)4(Me)2azasilagermane)

-
-
13463-40-6
iron pentacarbonyl

-
B
-
124686-68-6
((t-Bu)4(Me)2azasilagermane)iron tetracarbonyl

| Conditions | Yield |
|---|---|
| In benzene To a soln. of Ge compd. in benzene is added the carbonyl compd., suspn. is stirred for 12 h.; Recrystn. from toluene, NMR.; | A n/a B 97% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
38611-86-8
1,3-bis(diphenylstibino)propane

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2); soln. of Fe complex (2 equiv.) and Sb ligand in THF was stirred atroom temp. for 2 d; filtered; volatiles removed (vac.); | A n/a B 97% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
33446-84-3
1,1-dimethoxy-1-silacyclobutane

-
-
13463-40-6
iron pentacarbonyl

-
B
-
68046-55-9
2,2,2,2-tetracarbonyl-1,1-dimethoxy-1-sila-2-ferracyclopentane

| Conditions | Yield |
|---|---|
| In benzene addn. of 2 equiv. of Si-compd. to Fe-complex suspn. (held above freezingpoint), warming to 21°C, stirring (12 h); removal of Fe(CO)5 and solvent (vac.), distn. (1E-2 Torr, 30°C); elem. anal.; | A n/a B 96% |
| With carbon monoxide In not given N2-atmosphere; |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran reductive disproportionation, mechanism discussed;; IR; iron carbonyl not isolated;; | A 82% B 94% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In petroleum ether addn. of Fe2(CO)9 to soln. of Fe org. complex, stirring for 30 min at 40°C; filtering, chromy., recrystn. from petroleum ether; | A n/a B 92% |

-
-
122190-26-5
N(C6H4OCH3)C(O)(C(COOCH3))2C(CH3)C(CH3)N(C6H4OCH3)Fe(CO)3

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In acetone in a steel autoclave with glass insert, keeping at 75-90 °C, 4 d, under 180 bar of CO pressure; evapn. of the solvent and Fe(CO)5; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran N2, stirring for 6 h at room temp.;; evapd., dissolved in CH2Cl2/light petroleum 1/1, chromd. (alumina, CH2Cl2/light petroleum 1/1), evapd., elem. anal.; | A n/a B n/a C 88% |

-
-
2295-12-7
1,1-dimethylsilacyclobutane

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

-
B
-
17685-52-8
triiron dodecarbonyl

-
C
-
39114-08-4
2,2,2,2-tetracarbonyl-1,1-dimethyl-1-sila-2-ferracyclopentane

| Conditions | Yield |
|---|---|
| In benzene addn. of 2 equiv. of Si-compd. to Fe-complex suspn. (held above freezingpoint), warming to 21°C, stirring (24 h); removal of Fe(CO)5 and solvent (vac.), dissoln. in hexane, crystn. (-30°C, several days), filtration off of Fe3(CO)12, evapn. of filtrate(vac., low temp.), sublimation (1E-3 Torr, 0°C); elem. anal.; | A n/a B n/a C 86% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In diethyl ether N2, stirring for 5 h at ambient temp.; evapd., dissolved in CH2Cl2/light petroleum 1/2, separation by chromy. (Kieselgel, water-cooled, CH2Cl2/light petroleum 1/2, then CH2Cl2/thf 1/1), evapd., elem. anal.; | A n/a B 85% C n/a |


-
A
-
553-90-2
Dimethyl oxalate

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 byproducts: CO; introducing a soln. of Fe-complex in CD2Cl2 into NMR tube, maintaining temp. at 28°C under N2; monitoring by (13)C-NMR and gas chromatography; | A 15% B n/a C 85% |
| With carbon monoxide In dichloromethane-d2 introducing a soln. of Fe-complex in CH2Cl2 into an autoclave thermostated at 34°C, stirring for 2.5 h under CO pressure; monitoring by (13)C-NMR and gas chromatography, cooling, removal of solvent under vacuum, redissolution in CD2Cl2, monitoring by (13)C-NMR; |


-
-
13463-40-6
iron pentacarbonyl

-
B
-
35679-07-3, 14649-69-5
tetracarbonyl(triphenylphosphine)iron

| Conditions | Yield |
|---|---|
| With carbon monoxide In Petroleum ether thermal decompn. in petroleum ether at 70°C under 1 atm of CO; analyzed by IR; | A 0% B 0% C 80% |


| Conditions | Yield |
|---|---|
| A n/a B 77% |


| Conditions | Yield |
|---|---|
| In diethyl ether N2, stirring for 4 h at ambient temp.; evapd., dissolved in CH2Cl2/light petroleum 1/5, chromd. (Kieselgel, water-cooled, CH2Cl2/light petroleum 1/5, then 1/1), evapd., recrystd. from light petroleum at -78°C, elem. anal.; | A n/a B n/a C 76% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In diethyl ether N2 atmosphere; stirring suspn. of W-copmd. and Fe-compd. in Et2O (15 h), removal of Fe(CO)5, addn. of Se and Et2O, stirring (15 h); removal of volatiles (vac.), extn. (CH2Cl2 and petroleum ether), chromy. (Kieselgel, CH2Cl2/petroleum ether), concn. (vac.), cooling (-78°C); elem. anal.; | A n/a B 76% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
93923-00-3
μ3-arsenic-hexacarbonyltris(η5-cyclopentadienyl)trimolybdenum (3 Mo-Mo)

-
-
13463-40-6
iron pentacarbonyl

-
C
-
111769-94-9
μ4-arsenic-μ-carbonyl-diμ(Fe, Mo)-carbonyl-dicarbonylbis(η5-cyclopentadienyl)(dicarbonyliron){tricarbonyl(η5-cyclopentadienyl)molybdenum}dimolybdenum (2 Fe-Mo, Mo-Mo)

| Conditions | Yield |
|---|---|
| In toluene refluxing for 1.5 h; solvent and Fe(CO)5 removed in vac., residue dissolved in toluene; chromy. (silica gel) with hexane/toluene (1:1) yields Cp2Mo2(CO)6, then with toluene PhMe(MoCp(CO)2)2(AsMoCp(CO)3)Fe(CO)5 which is crystd. from CH2Cl2/hexane at -18°C; elem. anal.; | A n/a B n/a C 75% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In diethyl ether N2 atmosphere; stirring suspn. of W-copmd. and Fe-compd. in Et2O (15 h), removal of Fe(CO)5, addn. of Se and Et2O, stirring (15 h); removal of volatiles (vac.), extn. (CH2Cl2 and petroleum ether), chromy. (Kieselgel, CH2Cl2/petroleum ether), concn. (vac.), cooling (-78°C); | A n/a B 75% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In diethyl ether N2 atmosphere; stirring suspn. of W-copmd. and Fe-compd. in Et2O (15 h), removal of Fe(CO)5, addn. of Se and Et2O, stirring (15 h); removal of volatiles (vac.), extn. (CH2Cl2 and petroleum ether), chromy. (Kieselgel, CH2Cl2/petroleum ether), concn. (vac.), cooling (-78°C); elem. anal.; | A n/a B 74% |


| Conditions | Yield |
|---|---|
| With methyl iodide In cyclohexane byproducts: n-nonyl iodide, n-decane, CH3COC9H19; (N2); added n-tridecane, methyl iodide and cyclohexane to Fe-complex; stirred at room temp. for 3 h; cooled to -78°C; IR, GLPC, HPLC; | A 46% B 73% |
-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; Inert atmosphere; Schlenk technique; | A n/a B 72% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
30681-90-4
1,1,2-Trimethyl-1-silacyclobutane

-
-
13463-40-6
iron pentacarbonyl

-
B
-
17685-52-8
triiron dodecarbonyl

-
C
-
60104-88-3
2,2,2,2-tetracarbonyl-1,1,5-trimethyl-1-sila-2-ferracyclopentane

| Conditions | Yield |
|---|---|
| In benzene N2-atmosphere; slight excess of Si-compd., stirring (21°C, 24 h); evapn. (vac., cooling to just above freezing), dissoln. in hexane, standing at -30°C for several d, filtration off of Fe3(CO)12 (-30°C), evapn. of filtrate (low temp., vac.), distn. (0.001 Torr, probe at 0°C); elem. anal.; | A n/a B n/a C 71% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 16h; Schlenk technique; Darkness; Inert atmosphere; | A n/a B 71% |


-
-
13463-40-6
iron pentacarbonyl

-
C
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 byproducts: CO; introducing a soln. of Fe-complex in CD2Cl2 into NMR tube, maintaining temp. at 28°C under N2; monitoring by (13)C-NMR and gas chromatography; | A n/a B 70% C 30% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
50964-61-9
Methyl-bis-dimethylarsino-amin

-
-
13463-40-6
iron pentacarbonyl

-
B
-
101482-34-2
bis(dimethylarsino)methylaminoirontetracarbonyl

| Conditions | Yield |
|---|---|
| In diethyl ether (N2); arsenic-compd. in 40% excess; react. at 25°C for 16 h;; filtn., solvent is removed, destillation, elem. anal.;; | A n/a B 69% |

-
-
122501-48-8
3,4-bis(2',2,'6',6'-tetramethylpiperidino)-1,2-diphospha-3,4-dibora[1.1.0]bicyclobutane

-
-
15321-51-4
diiron nonacarbonyl

-
-
13463-40-6
iron pentacarbonyl

-
B
-
122575-20-6
2,4-bis(2',2',6',6'-tetramethylpiperidino)-1,3,2,4-diphosphadiborabicyclo{1.1.0}-butan-bis(tetracarbonyleisen)

| Conditions | Yield |
|---|---|
| In toluene stirring at room temp. under N2 for 1 day; evapn. (vac.) and recrystn. from Et2O; elem. anal.; | A n/a B 68% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
23800-57-9
1,3,5-triphenylpent-2-en-1,5-dione

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In benzene at 15-25 °C,for 4-20 h; | A n/a B 65% |
| In toluene at 15-25 °C,for 4-20 h; | A n/a B 65% |

-
-
13463-40-6
iron pentacarbonyl

-
-
56-34-8
tetraethylammonium chloride

| Conditions | Yield |
|---|---|
| With sodium amalgam In diethylene glycol Ar, soln. of Fe(CO)5 treated with Na#Hg, Fe(CO)5 added, stirred and heated to 150-160°C, continued for 3-4 h at 130-140°C, cooledto about 20°C, Rh-compound added, stirred for 30-40 min, filtered, 3-fold excess hexane added; decanted; ppt. washed (hexane); extd. (water); Et4NCl in H2O added; pptfiltered; washed (water); dried (vac., 20°C); recrystd. (CH2Cl2); elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In water Kinetics; Irradiation (UV/VIS); React. occurs at room temp.; | 99% |
| In water; acetone Kinetics; Irradiation (UV/VIS); React. occurs at room temp.; | 99% |
| In further solvent(s) Kinetics; Irradiation (UV/VIS); React. occurs at room temp. in perfluorodecaline or perfluoropolyether.; | 99% |

| Conditions | Yield |
|---|---|
| cobalt(II)-iodide * 4 H2O In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 0.5 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 99% |
| pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 94% |
| [(η(5)-C5H4Me)Fe(CO)2]2 In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 3 h; evapd. in vac., chromd., recrystd.; | 88% |
-
-
13463-40-6
iron pentacarbonyl

-
-
603-35-0
triphenylphosphine

-
-
35679-07-3, 14649-69-5
tetracarbonyl(triphenylphosphine)iron

| Conditions | Yield |
|---|---|
| cobalt(II)-iodide * 4 H2O In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 0.5 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 99% |
| CoBr2*3H2O In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 1.25 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 98% |
| cobalt(II) chloride dihydrate In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 2 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 83% |
| With trimethylamine-N-oxide In ethanol; hexane Kinetics; byproducts: CO2, (CH3)3N; mixing of a soln. of (CH3)3NO in C2H5OH and a soln. of PPh3 in n-C6H14 (at least 10 fold excess) with a soln. of Ru(CO)5 in n-C6H14 (compn. of mixed solvent: 1/2(v/v) C2H5OH/n-hexane) at different temp. in the range 11.3-33.9°C, vigorous shaking; detn. by IR; |
-
-
13463-40-6
iron pentacarbonyl

-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
12152-72-6
(cyclohexa-1,3-diene)iron tricarbonyl

| Conditions | Yield |
|---|---|
| With catalyst: PhCH=CH-CH=N-C6H4(OMe-4) In 1,4-dioxane Ar-atmosphere; refluxing soln. of Fe-complex, catalyst and cyclohexadiene (excess) for 45 h; evapn., chromy. (SiO2, pentane); | 99% |
| With catalyst: PhCH=CH-CH=N-C6H4(OMe-4) In 1,4-dioxane Ar-atmosphere; refluxing soln. of Fe-complex, catalyst and cyclohexadiene (excess) for 45 h, addn. of new portion of catalyst and diene, refluxing for 24 h; evapn., dissoln. (pentane), filtration (Celite), evapn.; | 89% |
| In hexane Irradiation (UV/VIS); Ar atmosphere; | 77% |
-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| With iodine In toluene Fe(CO)5 soln. added to ligand soln., I2 added, refluxed for 5 h; cooled, filtered, evapd.; solid residue after filtration extd. (CHCl3) mixed with previously obtained solid, soln. stirred with aq. NaOH for 4 h, org. layer evapd., dissolved in CH2Cl2, chromd. (Al2O3, hexane/CH2Cl2, CH2Cl2); | 99% |
-
-
13463-40-6
iron pentacarbonyl

-
-
344310-48-1
(CH3)2PCH2CH2(CH3)SiCH2CH2Si(CH3)CH2CH2P(CH3)2

-
-
84180-96-1
(CH3)2PCH2CH2(CH3)SiCH2CH2Si(CH3)CH2CH2P(CH3)2[Fe(CO)4]2

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CO; (N2 or vac.); 5 h; 150°C; dissolving in benzene; removal of insol. compounds; removal of solvent in vac.; elem. anal.; | 99% |
-
-
13463-40-6
iron pentacarbonyl

-
-
345652-78-0
((CH3)2PCH2CH2)(C6H5)SiCH2CH2Si(C6H5)(CH2CH2P(CH3)2)

-
A
-
84180-98-3
(CH3)2PCH2CH2(C6H5)SiCH2CH2Si(C6H5)CH2CH2P(CH3)2[Fe(CO)4]2

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CO; (N2 or vac.); 5 h; 150°C; dissolving in benzene; removal of insol. compounds; removal of solvent in vac.; elem. anal.; | A 99% B n/a |

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| With iodine In toluene Fe(CO)5 soln. added to ligand soln., I2 added, refluxed for 5 h; cooled, filtered, evapd.; solid residue after filtration extd. (CHCl3) mixed with previously obtained solid, soln. stirred with aq. NaOH for 4 h, org. layer evapd., dissolved in CH2Cl2, chromd. (Al2O3, hexane/CH2Cl2, CH2Cl2); | 99% |

| Conditions | Yield |
|---|---|
| In benzene-d6 Irradiation (UV/VIS); dry N2 or Ar; soln. of disulfide and Fe compd. (1:2 molar ratio) irradiated for 14 d, NMR control; crystd. on slow evapn., elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| In toluene at 130℃; Sonogashira Cross-Coupling; Sealed tube; | 98.2% |
-
-
13463-40-6
iron pentacarbonyl

-
-
35679-07-3, 14649-69-5
tetracarbonyl(triphenylphosphine)iron

| Conditions | Yield |
|---|---|
| With triphenylphosphine; tetrakis(triphenylphosphine)platinum In benzene byproducts: CO; under N2, refluxing soln. of metal carbonyl, org. compd. and Pt(PPh3)4 in benzene (molar ratio metal carbonyl/org. compd./Pt(PPh3)4 1:2:0.1, 5 h), cooling (room temp.); removal of solvent (vac.), dissoln. (CH2Cl2), chromy. (silica gel, CH2Cl2/hexane 1:1), recrystn. (CH2Cl2/hexane); | 98% |
| With triphenylphosphine; pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 97% |
| With triphenylphosphine; palladium(II) oxide In toluene byproducts: CO; 10 h, toluene under reflux, PdO catalyst;; not sepd., detected by IR-spectra;; | 96% |

| Conditions | Yield |
|---|---|
| [(η(5)-C5H4Me)Fe(CO)2]2 In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 2.0 h; evapd. in vac., chromd., recrystd.; | 98% |
| cyclopentadienyl iron(II) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.0 h; evapd. in vac., chromd., recrystd.; | 98% |
| pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 93% |
-
-
13463-40-6
iron pentacarbonyl

-
-
1486-28-8
diphenyl(methyl)phosphine

-
-
37410-36-9, 41206-81-9
Fe(CO)4PPh2Me

| Conditions | Yield |
|---|---|
| cyclopentadienyl iron(II) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.75 h; evapd. in vac., chromd., recrystd.; | 98% |
| [(η(5)-C5H4Me)Fe(CO)2]2 In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 98% |
| cobalt(II) chloride dihydrate In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 1 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 98% |
| pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 96% |
| cobalt(II)-iodide * 4 H2O In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 1.5 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 78% |
-
-
13463-40-6
iron pentacarbonyl

-
-
13246-32-7
ortho-phenylenebis(dimethylarsine)

-
A
-
56760-75-9
C6H4(As(CH3)2)2Fe(CO)4

-
B
-
15308-59-5, 56760-75-9
(CO)3Fe{(CH3)2AsC6H4As(CH3)2}

| Conditions | Yield |
|---|---|
| In neat (no solvent) tube was charged with ligand and Fe(CO)5, degassed and sealed under vac. (0.1 mmHg), tube was wrapped in foil and heated in an oil bath at 180°C for 7 h under N2, allowed to stand overnight at room temp., tube was frozen in liq. N2; solid residue was crushed, washed with ether under N2, taken up in CH2Cl2, soln. was filtered under N2, solvent was removed, chromd. under N2 on Florisil, eluted with benzene/hexane; | A n/a B 98% |

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Fe(CO)5 and tris(1,2-quinone mono-oximato)iron(III) heated under reflux in dry THF under nitrogen for 24 h; product filtered off, washed with THF and dried at 100°C/0.1 mmHg; elem. anal.; | 98% |
-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Fe(CO)5 and tris(1,2-quinone mono-oximato)iron(III) heated under reflux in dry THF under nitrogen for 24 h; product filtered off, washed with THF and dried at 100°C/0.1 mmHg; elem. anal.; | 98% |
-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In toluene (N2); reflux of mixt. for 3 days (125°C); filtn., residue is washed with toluene and cooled down to -30°C, soln. is decanted, crystals are washed with hexane, elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 98% |
| cyclopentadienyl iron(II) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 2.5 h; evapd. in vac., chromd., recrystd.; | 97% |
| [(η(5)-C5H4Me)Fe(CO)2]2 In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 2.25 h; evapd. in vac., chromd., recrystd.; | 95% |
-
-
13463-40-6
iron pentacarbonyl

-
-
84114-40-9
((CH3)2PCH2CH2)2SiCH2CH2CH2

-
-
84206-91-7
[Fe(CO)4]2((CH3)2PCH2CH2)2SiCH2CH2CH2

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CO; (N2 or vac.); 5 h; 140°C; dissolving in benzene; removal of insol. compounds; removal of solvent in vac.; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| In toluene at 25℃; for 18h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: C58H72FeN4O4Si2 In toluene at 20℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: iron pentacarbonyl In toluene at 20 - 100℃; Schlenk technique; Inert atmosphere; | 97.1% |

| Conditions | Yield |
|---|---|
| pentamethylcyclopentadienyliron(III) dicarbonyl dimer In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 1.25 h; evapd. in vac., chromd., recrystd.; | 97% |
| cobalt(II)-iodide * 4 H2O In toluene ligand and catalyst added to toluene, stirred soln. brought to reflux, Fe(CO)5 added to this soln., reflux continued for 1 h under N2; catalyst and excess ligand removed by chromy., solvent and excess Fe(CO)5 removed by evapn.; | 97% |
| [(η(5)-C5H4Me)Fe(CO)2]2 In toluene to boiling soln. of Fe(CO)5 and catalyst added ligand, heated for 2.0 h; evapd. in vac., chromd., recrystd.; | 96% |
-
-
13463-40-6
iron pentacarbonyl

-
-
431-94-7
N-chlorobistrifluoromethylamine

-
-
371-71-1
Perfluoro-2-azapropen

| Conditions | Yield |
|---|---|
| 0°C; | 97% |
| 0°C; | 97% |
-
-
13463-40-6
iron pentacarbonyl

-
-
758-43-0
N-bromobis(trifluoromethyl)amine

-
-
371-71-1
Perfluoro-2-azapropen

| Conditions | Yield |
|---|---|
| 0°C; | 97% |
| 0°C; | 97% |
-
-
13463-40-6
iron pentacarbonyl

-
-
118734-49-9
4,5-diethyl-2,5-dihydro-3-isopropenyl-2,2-dimethyl-1,2,5-selenasilaborole

-
A
-
118772-37-5
tricarbonyl(η4-4,5-diethyl-2,5-dihydro-3-isopropenyl-2,2-dimethyl-1,2,5-selenasilaborole)iron

-
B
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); Ar atmosphere, photolysis (125-W medium-pressure Hg lamp, 4.5 h); solvent removal (12 Torr), dissoln. (pentane), filtn., evapn., sublimation (70-80°C, 0.001 Torr); elem. anal.; | A 73% B 97% |

-
-
13463-40-6
iron pentacarbonyl

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Fe(CO)5 and tris(1,2-quinone mono-oximato)iron(III) heated ander reflux in dry THF under nitrogen for 24 h; product filtered off, washed with THF and dried at 100°C/0.1 mmHg; elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| In pentane under dry nitrogen; stirred 6 h at 0°C; washed with cold pentane, dried in a steam of N2; elem. anal.; | 97% |
Iron pentacarbonyl Chemical Properties
CAS: 13463-40-6
EINECS: 236-670-8
Following is the Molecular Structure of Iron pentacarbonyl (13463-40-6):
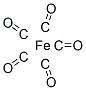
SMILES: C(#O)[Fe](C#O)(C#O)(C#O)C#O
InChI: InChI=1/5CO.Fe/c5*1-2;/rC5FeO5/c7-1-6(2-8,3-9,4-10)5-11
InChIKey: FYOFOKCECDGJBF-OZYZBPEXAA
Std.InChI: InChI=1S/5CO.Fe/c5*1-2
Std.InChIKey: FYOFOKCECDGJBF-UHFFFAOYSA-N
Molecular Formula: C5H10FeO5
Molecular Weight: 205.97
Melting Point: -20℃
Boiling Point: 103℃(lit.)
Flash Point: -15℃
Density: 1.49g/mL at 25℃(lit.)
Vapour Pressure: 35mm Hg( 25℃)
Refractive Index: n20/D 1.5196(lit.)
Iron pentacarbonyl Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | oral | 100mg/kg (100mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0591-1177, | |
| guinea pig | LCLo | inhalation | 140ppm/30M (140ppm) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0591-1177, | |
| guinea pig | LD50 | oral | 22mg/kg (22mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 415, 1943. | |
| mouse | LC50 | inhalation | 2190mg/m3/30M (2190mg/m3) | AMA Archives of Industrial Health. Vol. 19, Pg. 11, 1959. | |
| mouse | LD50 | intraperitoneal | 60mg/kg (60mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0591-1177, | |
| mouse | LD50 | oral | 62mg/kg (62mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 26(6), Pg. 52, 1982. | |
| rabbit | LCLo | inhalation | 250ppm/45M (250ppm) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 335, 1969. | |
| rabbit | LD50 | intravenous | 11mg/kg (11mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 415, 1943. | |
| rabbit | LD50 | oral | 12mg/kg (12mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 415, 1943. | |
| rabbit | LD50 | skin | 56mg/kg (56mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: CYANOSIS | National Technical Information Service. Vol. OTS0535885, |
| rabbit | LD50 | subcutaneous | 240mg/kg (240mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 415, 1943. | |
| rat | LC50 | inhalation | 10ppm/4H (10ppm) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Technical Information Service. Vol. OTS0535889, |
| rat | LD50 | oral | 25mg/kg (25mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0591-1177, |
Iron pentacarbonyl Consensus Reports
EPA Extremely Hazardous Substances List. Reported in EPA TSCA Inventory.
Iron pentacarbonyl Safety Profile
Hazard Codes: ![]() F Flammable,
F Flammable, ![]() T+ Very Toxic.
T+ Very Toxic.
Risk Statements: 11-24-26/28
R11: Highly Flammable.
R24: Toxic in contact with skin.
R26/28: Very Toxic by inhalation and if swallowed.
Safety Statements: 16-26-28-36/37/39-45
S16: Keep away from sources of ignition-No smoking.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer).
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
RIDADR: UN 1994 6.1/PG 1
WGK: Germany 2
RTECS: NO4900000
F: 10
TSCA: Yes
HazardClass: 6.1
PackingGroup: I
A poison by inhalation, skin contact, ingestion, subcutaneous, and intravenous routes. Inhalation causes dizziness, nausea, and vomiting. If continued, unconsciousness follows. Often there is a delayed reaction of chest pain, cough, and difficult breathing. There may be cyanosis and circulatory collapse. In fatal cases, death occurs from the fourth to eleventh day with pneumonitis and injury to kidneys, liver, and brain. Iron carbonyl is less toxic than nickel carbonyl.A very dangerous fire and moderate explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Warning: pyrophoric in air. Mixtures with nitrogen oxide explode above 50℃. Violent reaction with zinc + transition metal halides (e.g., cobalt halides, rhodium halides, ruthenium halides). Mixtures with acetic acid + water produce a pyrophoric powder. To fight fire, use water, foam, CO2, dry chemical.
Iron pentacarbonyl Standards and Recommendations
OSHA PEL: TWA 0.1 ppm (Fe); STEL 0.2 ppm
ACGIH TLV: TWA 0.1 ppm (Fe); STEL 0.2 ppm
DFG MAK: 0.1 ppm (0.81 mg/m3)
DOT Classification: 6.1; Label: Poison, Flammable Liquid
Iron pentacarbonyl Specification
Iron pentacarbonyl (13463-40-6),which also can be called for (Betab-5-11)-ironcarbonyl(Fe(CO)5) ; Fe(CO)5 ; Ferpentacarbonyle ; Ferpentacarbonyle(French) ; Iron carbonyl (Fe(CO)5) ; Iron carbonyl (Fe(CO)5),(tb-5-11)- ; Ironcarbonyl(Fe(CO)5) ; Ironcarbonyl(Fe(CO)5),(tb-5-11) . Iron pentacarbonyl (13463-40-6) is a yellow-orange to brown liquid and it's insoluble in water and denser than water.Be attention fo it's very toxic by inhalation, ingestion, and skin absorption.This chemical is a highly flammable and air sensitive.It's stable in dry air.
Related Products
- Iron
- Iron alloy, base
- Iron antimonide
- Iron difluoride
- Iron disulfide
- Iron fluoride (FeF3),trihydrate (8CI,9CI)
- Iron hydroxide
- Iron manganese trioxide
- Iron manganese zinc oxide
- Iron methanearsonate
- 13463-41-7
- 13463-43-9
- 13463-67-7
- 13463-71-3
- 134637-67-5
- 13464-19-2
- 13464-24-9
- 13464-35-2
- 13464-43-2
- 13464-44-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View