-
Name
Lamivudine
- EINECS 603-844-3
- CAS No. 134678-17-4
- Article Data59
- CAS DataBase
- Density 1.73 g/cm3
- Solubility 70g/L(temperature not stated)
- Melting Point 177 °C
- Formula C8H11N3O3S
- Boiling Point 475.4 °C at 760mmHg
- Molecular Weight 229.26
- Flash Point 241.3 °C
- Transport Information
- Appearance white crystalline powder
- Safety 26-36
- Risk Codes 63-36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Epivir; GR109714X
- PSA 115.67000
- LogP -0.01290
Synthetic route

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; ethanol at 0 - 20℃; for 4h; | 97.9% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 0℃; for 10h; | 95% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl (2R,5S)-5-[2-oxo-4-(phenylcarboxamido)-1,2-dihydro-1-pyrimidinyl]-1,3-oxathiolane-2-carboxylate With methanol at 40℃; Inert atmosphere; Stage #2: With sodium tetrahydroborate; dipotassium hydrogenphosphate; water; sodium hydroxide In methanol at 20℃; for 1h; diastereoselective reaction; | 95% |
-
-
147027-10-9
(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl (2R,5S)-5-(4-amino-2-oxo-1,2-dihydro-1-pyrimidinyl)-1,3-oxathialane-2-carbaxylate

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 0.5h; Ambient temperature; | 94% |
| With methanol; sodium tetrahydroborate; dipotassium hydrogenphosphate; sodium hydroxide In water at 20℃; for 0.055h; Flow reactor; | 94% |
| With sodium tetrahydroborate In ethanol | 83% |
-
-
173522-96-8
lamivudine salicyclic acid salt

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 50℃; for 1h; | 92.63% |
| With triethylamine In water; ethyl acetate at 25 - 50℃; for 5h; Product distribution / selectivity; | |
| With triethylamine In water; ethyl acetate at 45 - 50℃; for 4.5h; |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: 4-amino-1-[(2R,5S)-(2-hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one (S)-BINOL co-crystal With hydrogenchloride In water; ethyl acetate at 20℃; for 1h; pH=3 - 4; Stage #2: With sodium hydroxide In water pH=~ 7; Product distribution / selectivity; | 90% |
| With hydrogenchloride In water; ethyl acetate pH=2 - 3; Large scale reaction; optical yield given as %ee; | 70.02% |
| With hydrogenchloride In water; ethyl acetate pH=2 - 2.5; | |
| Stage #1: 4-amino-1-[(2R,5S)-(2-hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one (S)-BINOL co-crystal With hydrogenchloride In water; ethyl acetate at 20℃; for 0.0833333h; pH=3 - 4; Stage #2: With sodium hydroxide In water pH=6.8 - 7.2; Reagent/catalyst; | 7 g |
| In water; ethyl acetate at 2 - 45℃; Industrial scale; | 33.3 kg |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 70 - 75℃; Inert atmosphere; Industry scale; | 90% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 20℃; for 14h; Inert atmosphere; | 89% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In Isopropyl acetate; water at 23 - 27℃; for 10h; Product distribution / selectivity; | 88% |
-
-
1012053-56-3
(2R,5S)-5-(4"-amino-2"-oxo-pyrimidin-1"-yl)-1,3-oxathiolane-2-methyl-(2'S-isopropyl-5'R-methyl-1'R-cyclohexyl)-carbonic acid diester

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 0 - 25℃; for 3h; | 87.17% |
| With potassium carbonate In methanol at 0 - 25℃; for 2h; | 86% |
-
-
145985-97-3
(2R,5S)-1-<2-<<(tert-butyldiphenylsilyl)oxy>methyl>-1,3-oxathiolan-5-yl>cytosine

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 0.5h; Ambient temperature; | 75% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Ambient temperature; | 75% |
-
-
131086-21-0, 131086-22-1, 134678-17-4, 134680-32-3, 136846-20-3, 136891-12-8, 139757-68-9, 141434-39-1, 146726-78-5
(+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: (+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine With (S)-N-acetyl-2-phenylglycine In methanol; acetone at -30 - 0℃; for 48h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; Product distribution / selectivity; | 32% |
| Stage #1: (+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine With (S)-N-acetyl-2-phenylglycine In methanol; acetone at -30 - 0℃; for 48h; Resolution of racemate; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; Purification / work up; | 32% |
| Multi-step reaction with 3 steps 1: 95 percent / dimethylformamide / 20 °C 2: 50.6 percent / pyridine / acetonitrile / 48 h / 0 °C 3: 95 percent / K2CO3 / methanol / 10 h / 0 °C View Scheme |
-
-
143957-12-4
Benzoic acid (2R,5S)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolan-2-ylmethyl ester

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With Amberlite IRA400(OH) In ethanol Heating; |


-
B
-
134678-17-4
lamivudine

-
C
-
139757-68-9
(+)-(2R,5R)-1-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With Amberlit IRA400 In methanol Heating; Yield given. Yields of byproduct given; | |
| With Amberlit IRA400 In methanol Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |



-
B
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With ammonia In methanol Yield given. Title compound not separated from byproducts; |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 50.6 percent / pyridine / acetonitrile / 48 h / 0 °C 2: 95 percent / K2CO3 / methanol / 10 h / 0 °C View Scheme |
-
-
139757-74-7
(2R,5S)-1-<2-<<(tert-butyldiphenylsilyl)oxy>methyl>-1,3-oxathiolan-5-yl>-N4-acetylcytosine

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 73 percent / methanolic NH3 / 3 h / Ambient temperature 2: 75 percent / tetra-n-butylammonium fluoride / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 73 percent / NH3 / methanol / Ambient temperature 2: 75 percent / tetra-n-butylammonium fluoride / tetrahydrofuran / Ambient temperature View Scheme |
-
-
131086-21-0, 131086-22-1, 134678-17-4, 134680-32-3, 136846-20-3, 136891-12-8, 139757-68-9, 141434-39-1, 146726-78-5
(+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine


-
B
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: (+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine With (S)-N-acetyl-2-phenylglycine In ethanol at -30 - -20℃; for 48h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; Product distribution / selectivity; | A n/a B n/a |
| Stage #1: (+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine With O,O'-dibenzoyl-L-tartaric acid In methanol; acetone at -30 - 20℃; for 48h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; Product distribution / selectivity; | A n/a B n/a |
| Stage #1: (+/-)-cis-N-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine With (S)-N-acetyl-2-phenylglycine In methanol; acetone at -50 - 25℃; for 48h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; Product distribution / selectivity; | A n/a B n/a |
-
-
147027-10-9
(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl (2R,5S)-5-(4-amino-2-oxo-1,2-dihydro-1-pyrimidinyl)-1,3-oxathialane-2-carbaxylate

-
-
69-72-7
salicylic acid

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl (2R,5S)-5-(4-amino-2-oxo-1,2-dihydro-1-pyrimidinyl)-1,3-oxathiolane-2-carboxylate With sodium tetrahydroborate; dipotassium hydrogenphosphate In ethanol; water at 18 - 22℃; for 8 - 9h; Stage #2: salicylic acid In water at 10 - 82℃; |

-
A
-
134678-17-4
lamivudine

-
B
-
139757-68-9
(+)-(2R,5R)-1-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 20℃; |

-
A
-
134678-17-4
lamivudine

-
B
-
139757-68-9
(+)-(2R,5R)-1-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 20℃; |

-
A
-
134678-17-4
lamivudine

-
B
-
139757-68-9
(+)-(2R,5R)-1-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 20℃; |
-
-
1091585-31-7
(2R,5S)-5-(4'-acetamido-2'-oxo-pyrimidin-1'-yl)-1,3-oxathiolane-2-methyl-(4'-chloro)-benzoate

-
A
-
134678-17-4
lamivudine

-
B
-
139757-68-9
(+)-(2R,5R)-1-<2-(hydroxymethyl)-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 20℃; |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: (2R-cis)-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate With hydrogenchloride In ethanol; water at 20 - 50℃; Stage #2: With sodium hydroxide In ethanol; water at 5 - 50℃; pH=7.5; Product distribution / selectivity; | n/a |

-
-
35193-63-6, 35193-64-7, 39648-67-4, 50574-52-2
(S)-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: cis-(+/-)-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone; (S)-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate With sodium hydroxide In water at 20 - 45℃; Stage #2: With hydrogenchloride In ethanol; water at 20℃; Stage #3: With sodium hydroxide In ethanol; water at 5 - 50℃; pH=7.5; Product distribution / selectivity; | n/a |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In water; ethyl acetate at 22 - 30℃; for 4.5h; | |
| With triethylamine In ethanol; water at 18 - 80℃; | |
| With triethylamine In water; ethyl acetate at 22 - 30℃; for 4.5h; Product distribution / selectivity; |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water at 22 - 30℃; for 4.5h; Product distribution / selectivity; | |
| With triethylamine In ethanol; ethyl acetate at 47 - 80℃; Product distribution / selectivity; |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With pyridine; tetraphosphorus decasulfide at 22 - 42℃; for 10h; Inert atmosphere; | |
| With pyridine; tetraphosphorus decasulfide at 22 - 42℃; for 10h; Inert atmosphere; |
-
-
93-97-0
benzoic acid anhydride

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 24h; | 98% |
| In ethanol at 70℃; for 2.5h; Sealed tube; | 80% |
-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| Stage #1: lamivudine With diphenyl hydrogen phosphite In pyridine at 20℃; for 0.5h; Stage #2: With triethylamine In water | 95% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | 95% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
134678-17-4
lamivudine

-
-
956896-97-2
(-)-5'-O-(t-butyldimethylsilyl)-2',3'-dideoxy-3'-thiacytidine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 18h; | 95% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; |
-
-
134678-17-4
lamivudine

-
-
121-44-8
triethylamine

| Conditions | Yield |
|---|---|
| Stage #1: lamivudine With diphenyl hydrogen phosphite In pyridine for 0.5h; Stage #2: triethylamine With water In pyridine | 93% |
-
-
134678-17-4
lamivudine

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
145985-97-3
(2R,5S)-1-<2-<<(tert-butyldiphenylsilyl)oxy>methyl>-1,3-oxathiolan-5-yl>cytosine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 20℃; for 3h; Substitution; | 90% |
-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With sodium (¹²⁵I)iodide; 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril In aq. buffer at 60℃; for 0.166667h; pH=7; | 89% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 88% |
-
-
159593-90-5, 192506-86-8
cis-[RuCl2(triphenylphosphine)2(2,2′-bipyridine)]

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; dichloromethane for 24h; Reflux; Inert atmosphere; | 85% |

-
-
108-24-7
acetic anhydride

-
-
134678-17-4
lamivudine

-
-
158704-08-6
4-acetylamino-1-[(2'R,5'S)-2'-(hydroxymethyl)-1,3-oxathiolan-5'-yl]-1,2-dihydropyrimidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: lamivudine In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: acetic anhydride In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: lamivudine With potassium hexamethylsilazane In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: 4-cyano-N,N,N-trimethylanilinium trifluoromethansulfonate In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 3h; Inert atmosphere; Schlenk technique; | 82% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane; N,N-dimethyl-formamide at 60℃; for 7h; solid phase reaction; | 80% |

-
-
134678-17-4
lamivudine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; Inert atmosphere; Schlenk technique; | 75% |

| Conditions | Yield |
|---|---|
| With pyridine; dicyclohexyl-carbodiimide | 73% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0℃; for 3h; | 70% |
-
-
134678-17-4
lamivudine

-
-
105108-59-6
(2-(2-methoxyethoxy)ethyl) (4-nitrophenyl) carbonate

-
-
1240259-44-2
C14H21N3O7S

| Conditions | Yield |
|---|---|
| With 1-hydroxy-pyrrolidine-2,5-dione In N,N-dimethyl-formamide at 20℃; for 72h; | 63% |

| Conditions | Yield |
|---|---|
| Stage #1: Octanoic acid With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In dichloromethane; N,N-dimethyl-formamide at 10℃; for 1h; Stage #2: lamivudine In dichloromethane; N,N-dimethyl-formamide for 20h; | 56% |
-
-
134678-17-4
lamivudine

-
-
200720-60-1
12-azidododecanoyl chloride

-
-
1373400-17-9
(-)-N(4)-5'-di(12-azidododecanoyl)-2',3'-dideoxy-3'-thiacytidine

| Conditions | Yield |
|---|---|
| With dmap In benzene at 100℃; for 4h; | 55% |
-
-
134678-17-4
lamivudine

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| Stage #1: lamivudine With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; Inert atmosphere; | 55% |
Lamivudine History
Lamivudine (CAS NO.134678-17-4) was produce by Bernard Belleau while at work at Nghe Nguyen-Ga and McGill University at the Montreal-based IAF BioChem International, Inc. laboratories in 1989. Initially designed as an antiviral agent, the drug's effectiveness for treating HIV in combination with AZT was discovered by Yung-Chi (Tommy) Cheng at Yale University. The drug was later licensed to the British pharmaceutical company Glaxo for a 14 percent royalty.
Lamivudine (CAS NO.134678-17-4) was approved by the Food and Drug Administration (FDA) on November 17, 1995 for use with zidovudine (AZT) and again in 2002 as a once-a-day dosed medication. The fifth antiretroviral drug on the market, it was the last NRTI for three years while the approval process switched to protease inhibitors. According to the manufacturer's 2004 annual report, its patent will expireand in Europe in 2011 and in the United States in 2010 .
Lamivudine Standards and Recommendations
APPEARANCE: White to off-white crystalline powder
ASSAY: 98.0 - 102.0%
RESIDUE ON IGNITION: 0.25% max
LOSS ON DRYING: 0.5% max
OPTICAL ROTATION: -93° ~ -100°
HEAVY METALS: 20 ppm max
Lamivudine Specification
1. Introduction of Lamivudine
Lamivudine (CAS NO.134678-17-4) is a white crystalline powder. The IUPAC Name of this chemical is 4-amino-1-[(2S,5R)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one. Lamivudine belongs to Active Pharmaceutical Ingredients;Antivirals for Research and Experimental Use;Biochemistry;Chemical Reagents for Pharmacology Research;Nucleosides and their analogs;Nucleosides, Nucleotides & Related Reagents;Anti-virals;Intermediates & Fine Chemicals;Pharmaceuticals;API's.
Its Classification Code is Anti-HIV agents; Anti-Infective Agents; Anti-Retroviral Agents; Antiviral; Antiviral Agents; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors; Reverse transcriptase inhibitors. Lamivudine is soluble in water, sparingly soluble in methanol and slightly soluble in ethanol.
2. Properties of Lamivudine
Physical properties about Lamivudine are:
(1)Index of Refraction: 1.754; (2)Molar Refractivity: 54.14 cm3; (3)Molar Volume: 132.2 cm3; (4)Polarizability: 21.46×10-24 cm3; (5)Surface Tension: 79.3 dyne/cm; (6)Density: 1.73 g/cm3; (7)Flash Point: 241.3 °C; (8)Enthalpy of Vaporization: 85.17 kJ/mol; (9)Melting Point: 177 °C; (10)Boiling Point: 475.4 °C at 760 mmHg; (11)Vapour Pressure: 4.91E-11 mmHg at 25°C; (12)XLogP3: -0.9; (13)H-Bond Donor: 2; (14)H-Bond Acceptor: 4.
3. Structure Descriptors of Lamivudine
(1)Canonical SMILES: C1C(OC(S1)CO)N2C=CC(=NC2=O)N
(2)Isomeric SMILES: C1[C@@H](O[C@@H](S1)CO)N2C=CC(=NC2=O)N
(3)InChI: InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m1/s1
(4)InChIKey: JTEGQNOMFQHVDC-RQJHMYQMSA-N
(5)Smiles: c1cn(c(=O)nc1N)[C@@H]2CS[C@@H](O2)CO
4. Toxicity of Lamivudine
Oral Toxicity: Not expected to be toxic following ingestion.
Inhalation Toxicity: No studies have been conducted.
5. Safety information of Lamivudine
RTECS: UW7361333
Hazardous Substances Data: 134678-17-4(Hazardous Substances Data)
6. Uses of Lamivudine
Lamivudine has been used for treatment of chronic hepatitis B at a lower dose than for treatment of HIV. It improves the seroconversion of e-antigen positive hepatitis B and also improves histology staging of the liver. Long term use of lamivudine unfortunately leads to emergence of a resistant hepatitis B virus (YMDD) mutant. Despite this, lamivudine is still used widely as it is well tolerated. Lamivudine is a reverse transcriptase inhibitor and antiviral.
7. Production of Lamivudine
Selective 6-O-sulfonylation reaction of compound (I), followed by acetylation to give the compound (II) in 96.7% yield. The compound (II) in acetic acid as solvent, and 3 mol of hydrogen bromide / L acid (45%, w / v) reaction, brominated to give the compound (III), in 99% yield. Bromide (III) and 3.3moI. Xanthan ethyl potassium, in acetone under reflux, thio and cyclization; then subjected to hydrolysis with ammonia in methanol, to obtain the compound (IV), two step yield 72%. Compound (IV) was purified by column chromatography, as a crystalline solid. Compound (IV) with 1.4 moles of sodium periodate treatment, ring-opening to give a 2,3 - cis-diol; the formation of an aldehyde followed by reduction with sodium borohydride, and formed in the form of a ketal to protect the diol to give compound (V), 60% yield. The compound (V) silylation to protect the rest of the primary alcohol, and then stripped of the ketal to give the compound (VI), 63% yield. Lead tetraacetate to the diol compound (VI) oxide, and then to the Shop two pyridinium salt to further oxidation, to obtain compound (VII), this oxidation method does not affect the sulfur. The compound (VII) and then oxidation of the lead tetraacetate, to obtain the compound (VIII), in 66% yield [meter] with the compound (VI). The compound (VIII) and (IX), in dichloroethane, TMSOTf as Lewis acid catalyst, obtained by condensation of the compound (X), a 64% yield. Isomer thereof, in addition to the compound (X) at half the amount of the compound (X), they can be used silica gel chromatography to separate. The compound (X) with ammonia - methanol come acetyl, 73% yield; then tetra-n-butylammonium fluoride to de-silylation, i.e. to obtain naphthalene, 75% yield.
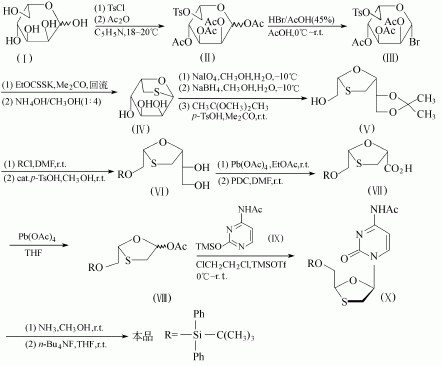
Or it can be got from Acetoxy-2-(diphenyl-t-butylsilyl-oxymethyl)-1,3-oxathiolane and 4-acetyl amino-2-trimethylsilyloxy pyrimidine dissolved in dichloroethane under the condensation of catalyzed Lewis acid.
Related Products
- Lamivudine
- Lamivudine salicylate
- 13467-82-8
- 134679-22-4
- 13468-00-3
- 134687-44-8
- 134690-32-7
- 134695-74-2
- 134698-42-3
- 13469-97-1
- 13469-98-2
- 13470-01-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View