-
Name
N-Cyclohexyl-2-benzothiazolesulfenamide
- EINECS 202-411-2
- CAS No. 95-33-0
- Article Data41
- CAS DataBase
- Density 1.26 g/cm3
- Solubility Insoluble in water
- Melting Point 93-100 °C
- Formula C13H16N2S2
- Boiling Point 410.4 °C at 760 mmHg
- Molecular Weight 264.415
- Flash Point 202 °C
- Transport Information UN 3077
- Appearance
- Safety 26-36/37/39-61-60-37-24
- Risk Codes 36/37/38-50/53-43
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  N
N
- Synonyms 2-(Cyclohexylaminothio)benzothiazole;Accelerator CZ;AccicureHBS;Banac CBS;Benzothiazyl-2-cyclohexylsulfenamide;CBS;CBS (accelerator);CBTS;Conac A;Conac S;Delac S;Ekagom CBS;N-Cyclohexyl-2-benzothiazolesulfenamide;N-Cyclohexyl-2-benzothiazolylsulphenamide;N-Cyclohexyl-2-benzothiazylsulfenamide;N-Cyclohexylbenzothiazole-2-sulphenamide;NSC 4809;Nocceler CZ-G;Nocceler CZ-P;Pennac CBS;Rhodifax 16;Accel CZ;2-Benzothiazolesulfenic acid N-cyclohexylamide;Sanceler CM;Royal CBTS;Sanceler CM-G;Santocure;Santocure CBS;Sulfenamide Ts;Sulfenax;SulfenaxCB;Sulfenax CB 30;Vulkacit C;Vulkacit CZ/C;Vulkacit CZ/EG;Vulkacit CZ/EG-C;
- PSA 78.46000
- LogP 4.61660
Synthetic route
-
-
120-78-5
di(benzothiazol-2-yl)disulfide

-
-
108-91-8
cyclohexylamine

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene | 100% |
-
-
2801-21-0
benzothiazole sulfenamide

-
-
108-91-8
cyclohexylamine

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| Stage #1: benzothiazole sulfenamide; cyclohexylamine In water at 30 - 40℃; for 3h; Stage #2: With sodium hydroxide In water at 35 - 85℃; for 1.5h; Reagent/catalyst; Concentration; | 99.5% |
| at 50℃; for 2h; Product distribution; |
-
-
108-91-8
cyclohexylamine

-
-
149-30-4
2-Mercaptobenzothiazole

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With (tetraphenylporphyrin)copper(II); oxygen In tetrachloromethane at 35℃; under 2625.26 Torr; for 0.5h; Pressure; Solvent; Temperature; Molecular sieve; Autoclave; | 97.2% |
| With oxygen In water at 60℃; under 3000.3 Torr; for 4h; Autoclave; | 96% |
| With oxygen In water at 60℃; under 2250.23 Torr; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With lead dioxide; potassium nitrate In dichloromethane at 38℃; for 2h; Temperature; | 97% |
-
-
108-91-8
cyclohexylamine

-
-
2492-26-4
sodium 2-mercaptobenzothiazole

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexylamine; sodium 2-mercaptobenzothiazole With sulfuric acid; dihydrogen peroxide In water at 40 - 45℃; for 3 - 5h; Alkaline conditions; Stage #2: With sodium hypochlorite In water for 0.166667 - 0.633333h; | 95.2% |
| With sulfuric acid; dihydrogen peroxide In water at 50℃; for 2h; Conversion of starting material; | 84% |
| With alkaline aqueous solution; chloro-air mixture |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 50℃; for 5.5h; Conversion of starting material; | 94.8% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite | 83% |
-
-
52185-81-6
N-chlorocyclohexanamine

-
-
2492-26-4
sodium 2-mercaptobenzothiazole

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With water |
-
-
108-91-8
cyclohexylamine

-
-
114698-09-8
benzothiazole-2-sulfenyl thiocyanate

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With diethyl ether |
-
-
40576-90-7
2-(Cyclohexylidenaminothio)benzothiazol

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether 2: diethyl ether View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; sodium hypochlorite 2: water View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / ethanol / 0.5 h / 70 - 72 °C 2: NaBH4 / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / ethanol / 0.5 h / 70 - 72 °C 2: NaBH4 / ethanol View Scheme |
-
-
762-04-9, 123-22-8
Diethyl phosphonate

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
A
-
32405-88-2
diethyl cyclohexylphosphoramidate

-
B
-
149-30-4
2-Mercaptobenzothiazole

| Conditions | Yield |
|---|---|
| A 83% B 100% |

-
-
691-96-3
diisopropyl phosphite

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
A
-
54480-52-3
diisopropyl N-cyclohexylphosphoramidate

-
B
-
149-30-4
2-Mercaptobenzothiazole

| Conditions | Yield |
|---|---|
| A 87% B 100% |

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
3264-02-6
bis-benzothiazol-2-ylsulfanyl-cyclohexyl-amine

| Conditions | Yield |
|---|---|
| With acetic anhydride In n-heptane at 68 - 70℃; for 1.41667h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Autoclave; Large scale; | 94.3% |
| With maleic anhydride | |
| With phthalic anhydride |
-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
104881-44-9
(N-cyclohexyl-2-benzothiazolesulfenamide)copper(II) chloride

| Conditions | Yield |
|---|---|
| In acetone addn. of the stoichiometric amount of the ligand to the Cu salt in acetone with stirring; filtn., washing with acetone and drying (vac.); elem. anal.; | 78% |
-
-
17166-16-4
phosphorous acid tetramethyldiamide methylester

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
A
-
615-22-5
2-methylmercaptobenzothiazole

-
B
-
2254-94-6
3-methylbenzothiazole-2-thione

-
C
-
130888-07-2
C11H16N3OPS2

| Conditions | Yield |
|---|---|
| at 100℃; under 10 Torr; for 4h; | A 20.4% B n/a C 70.2% |

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
68835-08-5
tetraethyl-thiophosphorodiamidic acid S-benzothiazol-2-yl ester

-
B
-
2757-92-8
2-(ethylthio)benzothiazole

-
C
-
16407-34-4
3-aethylbenzothiazolin-2-thion

| Conditions | Yield |
|---|---|
| at 120℃; under 20 Torr; for 4h; | A 65% B 22% C n/a |
| at 120℃; under 20 Torr; for 4h; | A 65% B 22% C n/a |

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
7789-45-9
copper(ll) bromide

-
-
104881-45-0
(N-cyclohexyl-2-benzothiazolesulfenamide)copper(II) bromide

| Conditions | Yield |
|---|---|
| In acetone addn. of the stoichiometric amount of the ligand to the Cu salt in acetone with stirring; filtn., washing with acetone and drying (vac.); elem. anal.; | 65% |
-
-
30463-59-3
tetrapropyl-diamidophosphoric acid ethyl ester

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
A
-
2757-92-8
2-(ethylthio)benzothiazole

-
B
-
16407-34-4
3-aethylbenzothiazolin-2-thion

-
C
-
68837-83-2
C19H32N3OPS2

| Conditions | Yield |
|---|---|
| at 130℃; under 20 Torr; for 3h; | A 21.4% B n/a C 63% |
| at 130℃; under 20 Torr; for 3h; | A 21.4% B n/a C 63% |

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

| Conditions | Yield |
|---|---|
| With ammonium carbamate; iodosylbenzene; acetic acid In isopropyl alcohol at 25℃; for 1h; Inert atmosphere; | 30% |
-
-
60-29-7
diethyl ether

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
103-71-9
phenyl isocyanate

-
-
886-59-9
N-cyclohexyl-N'-phenylurea

-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
584-84-9
2,4-Toluene diisocyanate

-
-
94091-13-1
1-Cyclohexyl-3-<3-isocyanato-p-tolyl>-harnstoff

| Conditions | Yield |
|---|---|
| In diethyl ether |
-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
101-68-8
di(4-isocyanatophenyl)methane

-
-
58890-25-8
1.1'--bis-<3-cyclohexyl>-harnstoff

| Conditions | Yield |
|---|---|
| In benzene |
-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
66021-69-0
benzothiazolyl hydrodisulphide

| Conditions | Yield |
|---|---|
| With hydrogen sulfide | |
| With tiolacetic acid |
-
-
95-33-0
N-(cyclohexyl)benzothiazole-2-sulfenamide

-
-
33405-92-4
2-benzothiazolylsulfenyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With acetic anhydride |
N-Cyclohexyl-2-benzothiazolesulfenamide Chemical Properties
Molecular Structure of N-Cyclohexyl-2-benzothiazolesulfenamide (CAS NO. 95-33-0):
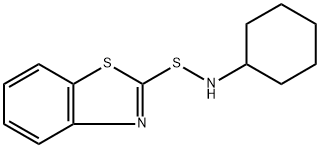
IUPAC Name: N-(1,3-Benzothiazol-2-ylsulfanyl)cyclohexanamine
Molecular Formula: C13H16N2S2
Molecular Weight: 264.409540 g/mol
Index of Refraction: 1.665
Molar Refractivity: 77.63 cm3
Molar Volume: 208.9 cm3
Surface Tension: 58.6 dyne/cm
Density: 1.26 g/cm3
Flash Point: 202 °C
Enthalpy of Vaporization: 66.27 kJ/mol
Boiling Point: 410.4 °C at 760 mmHg
Vapour Pressure: 6.04E-07 mmHg at 25 °C
Melting Point: 93-100 °C
Water Solubility: Insoluble
BRN: 0192376
EINECS: 202-411-2
Product Categories: Rubber Chemicals
N-Cyclohexyl-2-benzothiazolesulfenamide Toxicity Data With Reference
| 1. | orl-rat LD50:5300 mg/kg | JACTDZ Journal of the American College of Toxicology. 1 (1990),105. | ||
| 2. | ivn-mus LD50:32 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#02243 . |
N-Cyclohexyl-2-benzothiazolesulfenamide Consensus Reports
Reported in EPA TSCA Inventory.
N-Cyclohexyl-2-benzothiazolesulfenamide Safety Profile
Safety Information of N-Cyclohexyl-2-benzothiazolesulfenamide (CAS NO. 95-33-0):
Hazard Codes: Xi  ,N
,N 
Risk Statements: 36/37/38-50/53-43
R36/37/38:Irritating to eyes, respiratory system and skin
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
R43:May cause sensitization by skin contact
Safety Statements: 26-36/37/39-61-60-37-24
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S61:Avoid release to the environment. Refer to special instructions / safety data sheets
S60:This material and its container must be disposed of as hazardous waste
S37:Wear suitable gloves
S24:Avoid contact with skin
RIDADR: 3077
RTECS: DL6250000
HazardClass: 9
PackingGroup: III
A poison by intravenous route. Questionable carcinogen with experimental tumorigenic data. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and NOx.
N-Cyclohexyl-2-benzothiazolesulfenamide Specification
N-Cyclohexyl-2-benzothiazolesulfenamide with cas registry number of 95-33-0 is also called 2-(Cyclohexylaminothio)benzothiazole ; 2-Benzenethiazolesulfenamide, N-cyclohexyl- ; 2-Benzothiazolesulfenamide, N-cyclohexyl- ; N-Cyclohexyl-2-benzothiazylsulfenamide ; N-Cyclohexylbenzothiazole-2-sulphenamide ; AI3-16782 ; Accelerator CZ ; Accicure HBS ; Benzothiazyl-2-cyclohexylsulfenamide ; CBS ; CCRIS 4910 ; Conac A ; Conac H ; Conac S ; Curax ; Cyclohexyl benzothiazolesulfenamide ; Delac S ; Durax ; Ekagom CBS ; HSDB 2868 ; N-Cyclohexyl-2-benzothiazylsulfenamide ; N-Cyclohexylbenzothiazole-2-sulphenamide ; NSC 4809 ; Nocceler CZ ; Pennac CBS ; Rhodifax 16 ; Royal CBTS ; Sanceler CM-PO ; Santocure ; Santocure Pellets ; Santocure Powder ; Santocure vulcanization accelerator ; Soxinol CZ ; Sulfenamide TS ; Sulfenax ; Sulfenax CB ; Sulfenax CB 30 ; Sulfenax CB/K ; Sulfenax TsB ; Thiohexam ; Vulcafor CBS ; Vulcafor HBS ; Vulkacit C ; Vulkacit CZ ; Vulkacit CZ/C ; Vulkacit CZ/K ; Vulkacite CZ .
Related Products
- N-Cyclohexyl-2-benzothiazolesulfenamide
- 95333-14-5
- 95335-46-9
- 95337-96-5
- 95340-93-5
- 95-34-1
- 953410-86-1
- 953411-10-4
- 953414-75-0
- 95356-83-5
- 95360-22-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View