-
Name
diallyl tetrasulphide
- EINECS 219-485-7
- CAS No. 2444-49-7
- Article Data8
- CAS DataBase
- Density 1.211 g/cm3
- Solubility
- Melting Point
- Formula C6H10S4
- Boiling Point 279.2 °C at 760 mmHg
- Molecular Weight 210.409
- Flash Point 120.9 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Allyltetrasulfide (7CI);Tetrasulfide, di-2-propenyl (9CI);Tetrasulfide, diallyl(8CI);Diallyl tetrasulfide;ICD 1585;
- PSA 101.20000
- LogP 4.03620
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfur; potassium hydroxide; water In tetrahydrofuran for 2h; Ambient temperature; | A 30% B 10% |

| Conditions | Yield |
|---|---|
| With diethyl ether; sodium ethanolate beim Behandeln anschliessend mit Dischwefeldichlorid in Petrolaether; |
-
-
2179-57-9
diallyl disulphide

-
A
-
62488-53-3
3-vinyl-4H-<1,2>-dithiin

-
B
-
592-88-1
diallyl sulphide

-
C
-
2050-87-5
diallyl trisulfide

-
D
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 50℃; for 168h; Further byproducts given; | A 0.7 % Chromat. B 3 % Chromat. C 9.7 % Chromat. D 0.47 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

-
D
-
820-30-4
5-methyl-4,7-dithiadeca-1,9-diene

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.45 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

-
D
-
80028-57-5
2‐ethenyl‐4H‐1,3‐dithiine

| Conditions | Yield |
|---|---|
| at 50℃; for 168h; Further byproducts given; | A 3 % Chromat. B 9.7 % Chromat. C 0.47 % Chromat. D 0.3 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.68 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.42 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.86 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Yield given. Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D n/a |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Yield given. Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D n/a |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 2.3 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.2 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.7 % Chromat. |

-
-
2179-57-9
diallyl disulphide

-
A
-
592-88-1
diallyl sulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 80℃; for 240h; Further byproducts given; | A 20 % Chromat. B 18 % Chromat. C 5.5 % Chromat. D 0.25 % Chromat. |

-
-
106-95-6
allyl bromide

-
A
-
2179-57-9
diallyl disulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| With polysulfide; tetraethylammonium perchlorate In N,N-dimethyl-formamide in situ electrochemical generation of polysulfide from carbon-sulfur cathode; Title compound not separated from byproducts; |

-
-
107-05-1
3-chloroprop-1-ene

-
A
-
2179-57-9
diallyl disulphide

-
B
-
592-88-1
diallyl sulphide

-
C
-
2050-87-5
diallyl trisulfide

-
D
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| Stage #1: With sodium sulfide; sodium hydroxide; sulfur; polyethylene glycol In water at 45 - 60℃; under 1173.78 Torr; Stage #2: 3-chloroprop-1-ene In water at 45 - 50℃; under 915.2 - 4897.34 Torr; Product distribution / selectivity; continuous process; | |
| Stage #1: With sodium sulfide; sodium hydroxide; sulfur In water at 45 - 60℃; under 1173.78 Torr; Stage #2: 3-chloroprop-1-ene In water at 45 - 50℃; under 915.2 - 4897.34 Torr; Product distribution / selectivity; continuous process; |

-
-
539-86-6
allicin

-
A
-
2179-57-9
diallyl disulphide

-
B
-
592-88-1
diallyl sulphide

-
C
-
2050-87-5
diallyl trisulfide

-
D
-
2444-49-7
diallyl tetrasulfide

-
E
-
118686-45-6
bis-2-propenyl pentasulfide

| Conditions | Yield |
|---|---|
| With sulfur; dibutylamine at 60 - 70℃; for 1h; Product distribution / selectivity; |

-
-
539-86-6
allicin

-
A
-
2179-57-9
diallyl disulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

| Conditions | Yield |
|---|---|
| at 60 - 70℃; for 1h; Product distribution / selectivity; |

-
-
539-86-6
allicin

-
A
-
2179-57-9
diallyl disulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

-
D
-
118686-45-6
bis-2-propenyl pentasulfide

| Conditions | Yield |
|---|---|
| at 70℃; for 1h; Product distribution / selectivity; | |
| With sulfur at 60 - 70℃; for 1h; Product distribution / selectivity; |

-
-
539-86-6
allicin

-
A
-
2179-57-9
diallyl disulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

-
D
-
118686-45-6
bis-2-propenyl pentasulfide

-
E
-
137443-18-6
diallyl hexasulfide

| Conditions | Yield |
|---|---|
| With sulfur at 60 - 70℃; for 1h; Product distribution / selectivity; |

-
-
539-86-6
allicin

-
A
-
2179-57-9
diallyl disulphide

-
B
-
2050-87-5
diallyl trisulfide

-
C
-
2444-49-7
diallyl tetrasulfide

-
D
-
118686-45-6
bis-2-propenyl pentasulfide

-
E
-
137443-18-6
diallyl hexasulfide

-
F
-
139693-24-6
diallyl heptasulfide

| Conditions | Yield |
|---|---|
| With sulfur at 70℃; for 1h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| at 70℃; for 1h; Product distribution / selectivity; |
-
-
539-86-6
allicin

-
A
-
2050-87-5
diallyl trisulfide

-
B
-
2444-49-7
diallyl tetrasulfide

-
C
-
118686-45-6
bis-2-propenyl pentasulfide

-
D
-
137443-18-6
diallyl hexasulfide

-
E
-
139693-24-6
diallyl heptasulfide

| Conditions | Yield |
|---|---|
| With sulfur at 70℃; for 1h; Product distribution / selectivity; |

-
-
539-86-6
allicin

-
A
-
2050-87-5
diallyl trisulfide

-
B
-
2444-49-7
diallyl tetrasulfide

-
C
-
118686-45-6
bis-2-propenyl pentasulfide

-
D
-
139693-24-6
diallyl heptasulfide

| Conditions | Yield |
|---|---|
| With sulfur at 70℃; for 1h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| In ethanol Electrochem. Process; diallyltetrasulfide added to a soln. of CuBr2*2H2O in EtOH, placed into glass reservoir, copper electrodes mounted via air tight seal, system maintained for 20 h under an applied tension of alternating current (50 Hz, 0.2 V, 0.1 mA); crystn. on electrodes; |
Tetrasulfide,di-2-propen-1-yl Specification
The Tetrasulfide,di-2-propen-1-yl, with CAS registry number 2444-49-7, has the systematic name of diprop-2-en-1-yltetrasulfane. Besides this, it is also called Bis(2-propenyl) pertetrasulfide. And the chemical formula of this chemical is C6H10S4. What's more, its EINECS is 219-485-7.
Physical properties of Tetrasulfide,di-2-propen-1-yl: (1)ACD/LogP: 5.62; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.62; (4)ACD/LogD (pH 7.4): 5.62; (5)ACD/BCF (pH 5.5): 10930.74; (6)ACD/BCF (pH 7.4): 10930.74; (7)ACD/KOC (pH 5.5): 27067.87; (8)ACD/KOC (pH 7.4): 27067.87; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 101.2 Å2; (13)Index of Refraction: 1.623; (14)Molar Refractivity: 61.28 cm3; (15)Molar Volume: 173.6 cm3; (16)Polarizability: 24.29×10-24cm3; (17)Surface Tension: 45.7 dyne/cm; (18)Density: 1.211 g/cm3; (19)Flash Point: 120.9 °C; (20)Enthalpy of Vaporization: 49.7 kJ/mol; (21)Boiling Point: 279.2 °C at 760 mmHg; (22)Vapour Pressure: 0.00692 mmHg at 25°C.
Preparation: this chemical can be prepared by 3-chloro-propene. This reaction will need reagents S8, KOH and solvent tetrahydrofuran. The reaction time is 2 hour(s). The yield is about 30%.
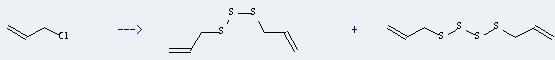
You can still convert the following datas into molecular structure:
(1)SMILES: S(SSSC\C=C)C/C=C
(2)InChI: InChI=1/C6H10S4/c1-3-5-7-9-10-8-6-4-2/h3-4H,1-2,5-6H2
(3)InChIKey: RMKCQUWJDRTEHE-UHFFFAOYAZ
(4)Std. InChI: InChI=1S/C6H10S4/c1-3-5-7-9-10-8-6-4-2/h3-4H,1-2,5-6H2
(5)Std. InChIKey: RMKCQUWJDRTEHE-UHFFFAOYSA-N
Related Products
- Tetrasulfide, dioctyl
- Tetrasulfide,bis(dibutoxyphosphinothioyl) (9CI)
- Tetrasulfide,bis(p-tolylsulfonyl) (6CI,8CI)
- Tetrasulfide,bis(thiocarboxy) (9CI)
- Tetrasulfide,bis[(1-piperidinylamino)thioxomethyl] (9CI)
- Tetrasulfide,di-2-propen-1-yl
- 24445-35-0
- 2444-68-0
- 24447-99-2
- 24448-20-2
- 2444-89-5
- 2445-07-0
- 24455-93-4
- 2445-67-2
- 2445-69-4
- 24457-21-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View