-
Name
Vinyl acetate
- EINECS 203-545-4
- CAS No. 108-05-4
- Article Data171
- CAS DataBase
- Density 0.93 g/cm3
- Solubility 23 g/L (20 °C) in water
- Melting Point -93 °C
- Formula C4H6O2
- Boiling Point 72.499 °C at 760 mmHg
- Molecular Weight 86.0904
- Flash Point 20°F
- Transport Information UN 1301 3/PG 2
- Appearance colourless mobile liquid
- Safety 16-23-29-33-45-36/37-7
- Risk Codes 11-39/23/24/25
-
Molecular Structure
-
Hazard Symbols
 F,
F, T
T
- Synonyms Acetic acid vinylester (8CI);1-Acetoxyethylene;Acetic acid, ethenyl ester;Acetoxyethene;Acetoxyethylene;Ethenyl acetate;NSC 8404;SN 12T;Vinyl A monomer;Vinylacetate;
- PSA 26.30000
- LogP 0.69300
Synthetic route

| Conditions | Yield |
|---|---|
| With acetic anhydride; benzenesulfonic acid at 140℃; under 1875.19 Torr; for 0.75h; Inert atmosphere; | 65.05% |
| With acetic anhydride; benzenesulfonic acid at 142℃; under 3000.3 Torr; for 0.75h; Inert atmosphere; | 64.47% |
| With acetic anhydride; benzenesulfonic acid at 143℃; under 3000.3 Torr; for 0.666667h; Inert atmosphere; | 64.57% |

| Conditions | Yield |
|---|---|
| With acetic anhydride; benzenesulfonic acid at 145℃; under 2850.29 Torr; for 0.75h; Inert atmosphere; | 63.51% |
| With lithium iodide | |
| With acetic anhydride; lithium iodide | |
| at 50 - 200℃; under 11400.8 Torr; |
-
-
764-78-3
ethylene glycol divinyl ether

-
-
507-02-8
acetyl iodide

-
A
-
108-05-4
vinyl acetate

-
B
-
593-66-8
vinyliodide

-
C
-
627-10-1
2-iodoethyl acetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 10℃; | A 42.8% B 40.5% C 54.9% |

-
-
74-85-1
ethene

-
-
64-19-7
acetic acid

-
A
-
108-05-4
vinyl acetate

-
B
-
542-59-6
2-hydroxyethyl acetate

-
C
-
111-55-7
ethylene glycol diacetate

| Conditions | Yield |
|---|---|
| With Pd(MeCN)2Cl(NO2) at 25℃; under 2068.6 Torr; for 4h; Product distribution; various catalysts, times, additives, O2 atmosphere, with 18O containing complex; | A 20% B 50% C 7% |

-
-
111-34-2
-butyl vinyl ether

-
-
507-02-8
acetyl iodide

-
A
-
108-05-4
vinyl acetate

-
B
-
542-69-8
1-iodo-butane

-
C
-
593-66-8
vinyliodide

-
D
-
123-86-4
acetic acid butyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 10℃; | A 11.8% B 12.8% C 35.8% D 39.8% |

-
-
74-85-1
ethene

-
-
127-08-2
potassium acetate

-
-
64-19-7
acetic acid

-
A
-
108-05-4
vinyl acetate

-
B
-
124-38-9
carbon dioxide

-
C
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With oxygen under 9120.61 Torr; for 16h; | A n/a B 11.57% C 0.151% |
| With oxygen under 9120.61 Torr; for 16h; | A n/a B 9.45% C 0.061% |
| With oxygen under 9120.61 Torr; for 16h; | A n/a B 9.1% C 0.049% |

-
-
36597-97-4
β,β-dichlorovinyl acetate

-
A
-
108-05-4
vinyl acetate

-
B
-
103659-54-7
Z-2-chlorovinyl acetate

-
C
-
10138-83-7, 103659-54-7
E-2-chlorovinyl acetate

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride for 0.5h; Ambient temperature; Irradiation; | A 2% B n/a C n/a |

-
-
15568-57-7
1-acetoxy-2-(ethyl-acetyl-amino)-ethane

-
A
-
108-05-4
vinyl acetate

-
B
-
3195-79-7
N-vinyl-N-ethyl-acetamide

-
C
-
625-50-3
N-ethylacetamide

| Conditions | Yield |
|---|---|
| at 490℃; Erhitzung; |


| Conditions | Yield |
|---|---|
| With copper (I) acetate; benzenesulfonic acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
542-10-9
ethylidene diacetate

-
-
142-71-2
copper diacetate

-
-
108-24-7
acetic anhydride

-
-
98-11-3
benzenesulfonic acid

-
A
-
108-05-4
vinyl acetate

-
B
-
75-07-0
acetaldehyde


| Conditions | Yield |
|---|---|
| With sodium bei Siedetemperatur; |

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
108-22-5
Isopropenyl acetate

-
-
2308-54-5
acetoxysulfonic acid

-
-
75-07-0
acetaldehyde

-
A
-
108-05-4
vinyl acetate

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| at 120℃; |


| Conditions | Yield |
|---|---|
| With 2-sulfo-acetoacetic acid at 120℃; |

| Conditions | Yield |
|---|---|
| With pyridine | |
| With N,N-dimethyl-aniline |

| Conditions | Yield |
|---|---|
| at 200 - 220℃; Leiten ueber mit Zink-Salzen impraegnierter Kohle; | |
| at 200 - 220℃; Leiten ueber mit Cadmium-Salzen impraegnierter Kohle; | |
| With complex salts of BF3; boron trifluoride |

| Conditions | Yield |
|---|---|
| With acetic anhydride at 40 - 60℃; under 3800 Torr; |


| Conditions | Yield |
|---|---|
| With mercury(II) sulfate; acetic acid at 60 - 100℃; |
-
-
74-85-1
ethene

-
A
-
108-05-4
vinyl acetate

-
B
-
542-59-6
2-hydroxyethyl acetate

-
C
-
542-10-9
ethylidene diacetate

-
D
-
111-55-7
ethylene glycol diacetate

-
E
-
107-21-1
ethylene glycol

-
F
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With sodium nitrate; oxygen; sodium chloride; palladium dichloride In acetic acid for 1h; Product distribution; Ambient temperature; presence of sodiun acetate; various pressure: 0.1 to 1.0 MPa; various temperature: 50 to 95 deg C; |

-
-
79-20-9
acetic acid methyl ester

-
-
201230-82-2
carbon monoxide

-
A
-
108-05-4
vinyl acetate

-
B
-
542-10-9
ethylidene diacetate

-
C
-
108-24-7
acetic anhydride

-
D
-
75-07-0
acetaldehyde

-
E
-
64-19-7
acetic acid

-
F
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| With ether-phosphane-silyloxy-rhodium; phosphane-silyloxy-platinum at 130℃; under 37503 Torr; for 5h; Product distribution; Mechanism; var. times and temp.; other rhodium complexes; |

-
-
927-68-4
bromoethyl acetate

-
A
-
108-05-4
vinyl acetate

-
B
-
111-55-7
ethylene glycol diacetate

-
C
-
628-67-1
butane-1,4-diol diacetate

-
D
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With nickel(II) salen; tetrabutylammonium tetrafluoroborate In acetonitrile Mechanism; electrolysis; other alkyl bromides also in the presence of activated olefins; | A 10 % Chromat. B 21 % Chromat. C 4 % Chromat. D 10 % Chromat. |
| With nickel(II) salen; tetrabutylammonium tetrafluoroborate In acetonitrile electrolysis; | A 10 % Chromat. B 21 % Chromat. C 4 % Chromat. D 10 % Chromat. |


| Conditions | Yield |
|---|---|
| at 682.6℃; Kinetics; |
-
-
1871-17-6
7-acetoxy-2,3:5,6-dibenzobicyclo<2.2.2>octa-2,5-diene

-
A
-
108-05-4
vinyl acetate

-
B
-
120-12-7
anthracene

| Conditions | Yield |
|---|---|
| In diphenylether at 250℃; Rate constant; |
-
-
64-19-7
acetic acid

-
-
74-86-2
acetylene

-
A
-
108-05-4
vinyl acetate

-
B
-
542-10-9
ethylidene diacetate

-
C
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With mercury(II) diacetate; toluene-4-sulfonic acid at 85℃; for 0.233333h; Kinetics; Mechanism; Product distribution; |


| Conditions | Yield |
|---|---|
| With methanolate In gas | |
| With methanolate In gas Product distribution; also with base F(1-); |
-
-
108-05-4
vinyl acetate

-
-
24442-57-7
1,2-dibromoethyl acetate

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane | 100% |
| With propane 3-bromo-1-(triphenylphosphonium) tribromide In dichloromethane at 20℃; for 0.5h; | 90% |
| With bromine for 3h; | 62% |

| Conditions | Yield |
|---|---|
| at 90℃; for 1h; microwave irradiation; | 100% |
| With immobilization of Candida cylindracea lipase In hexane at 55℃; for 15h; | 99% |
| With 1-(3-sulfopropyl)pyridinium phosphotungstate In neat (no solvent) at 100℃; for 0.5h; Microwave irradiation; | 91% |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With pseudomonas fuorescens lipase immobilized on multiwall carbon nano-tubes at 50℃; for 5h; Green chemistry; | 99% |
| With sulfuric acid beim Erhitzen; | |
| With aluminium trichloride at 105℃; | |
| In diethyl ether at 35℃; Candida cylindracea lipase; |

| Conditions | Yield |
|---|---|
| With Candida cylindracea lipase In hexane for 4h; | 100% |
| novozyme 435 In acetonitrile at 20℃; for 6h; Enzymatic reaction; | 99% |
| iodine at 20℃; for 2h; | 95% |
| With porcine pancreatic lipase (PPL, Type II) In tetrahydrofuran at 42 - 45℃; for 48h; Acetylation; | 80% |
| With Pseudomonas cepacia PS lipase In di-isopropyl ether at 25℃; for 0.5h; |
-
-
108-05-4
vinyl acetate

-
-
501-94-0
p-hydroxyphenethyl alcohol

-
-
58556-55-1
2-(4-hydroxyphenyl)ethyl acetate

| Conditions | Yield |
|---|---|
| With Candida cylindracea lipase In hexane for 4h; | 100% |
| With Candida antartica lipase B In tetrahydrofuran at 20℃; for 4h; Enzymatic reaction; chemoselective reaction; | 100% |
| With 1,3-dichlorotetrabutyldistannoxane In tetrahydrofuran at 30℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With Candida cylindracea lipase In hexane for 6h; | 100% |
| With porcine pancreatic lipase (PPL, Type II) In tetrahydrofuran at 42 - 45℃; for 120h; Acetylation; | 95% |
| With Pseudomonas cepacia PS lipase In di-isopropyl ether at 25℃; for 0.25h; |

| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst In dichloromethane at 170℃; for 8h; | 100% |
-
-
108-05-4
vinyl acetate

-
-
2612-30-8
2-benzyl-1,3-propanediol

-
-
110270-49-0
(R)-3-acetoxy-2-benzyl-1-propanol

| Conditions | Yield |
|---|---|
| Lipase P; | 100% |
| With Pseudomonas cepacia lipase on epoxysilica at 20℃; for 0.5h; Acetylation; | 100% |
| With 3 A molecular sieve; lipase Amono P supported on Celite In various solvent(s) at 25℃; for 5.7h; Acylation; | 95% |

| Conditions | Yield |
|---|---|
| With Candida cylindracea lipase In hexane for 4h; | 100% |
| Y5(OiPr)13O at 20℃; for 18h; Acetylation; transesterification; | 93% |
| With Pseudomonas cepacia PS lipase In di-isopropyl ether at 25℃; for 0.5h; |
-
-
108-05-4
vinyl acetate

-
-
30067-00-6, 84056-03-1, 86603-47-6, 125354-93-0, 125354-94-1, 127419-59-4, 131064-04-5, 142926-03-2
methyl (2RS,3RS)-2-hydroxy-3-(p-methoxyphenyl)-3-(o-nitrophenylthio)propanoate

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 22℃; for 48h; lipase from Pseudomonas cepacia; | 100% |
-
-
108-05-4
vinyl acetate

-
-
152961-72-3
2-(<1,1'-biphenyl>-4-ylmethyl)-1,3-propanediol

-
-
152961-75-6
(R)-3-Hydroxy-2-(4-phenylbenzyl)propyl acetate

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol In di-isopropyl ether; water for 2h; Ambient temperature; lipase PS (Pseudomonas sp.); | 100% |

| Conditions | Yield |
|---|---|
| With 1,3-dicyclohexylimidazolium-2-thiocarboxylate In tetrahydrofuran at 80℃; for 2h; Inert atmosphere; | A 100% B n/a |
| With lipase from Pseudomonas Cepacia In benzene at 35℃; other ω-substituted-1-alkanols, var. solvents; kinetic parameters of transesterification; |


| Conditions | Yield |
|---|---|
| Y5(OiPr)13O at 20℃; for 42h; Acetylation; transesterification; | 100% |
| With dilithium tetra(tert-butyl)zincate In tetrahydrofuran at 25℃; for 1h; Reagent/catalyst; Solvent; Time; Temperature; Inert atmosphere; | 100% |
| With P(MeNCH2CH2)3N In tetrahydrofuran for 2h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With N,N'-bismesityl-imidazol-2-ylidene In tetrahydrofuran at 20℃; for 1h; | 99% |
| 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene In tetrahydrofuran at 20℃; for 1h; | 99% |
-
-
108-05-4
vinyl acetate

-
-
208586-48-5
(E)-(S)-6-(4-Methoxy-benzyloxy)-2-methyl-hex-2-ene-1,4-diol

-
-
208586-60-1
Acetic acid (E)-(S)-4-hydroxy-6-(4-methoxy-benzyloxy)-2-methyl-hex-2-enyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 24h; PFL enzyme; | 100% |
-
-
108-05-4
vinyl acetate

-
A
-
6089-71-0
(11S)-3β,6α-dihydroxy-eudesman-12-oic acid-6-lactone

-
B
-
74493-56-4
(11S)-3β-acetoxy-6α-hydroxy-4βH-eudesman-12-oic acid-lactone

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase at 40℃; for 24h; Heating; | A 70 mg B 100% |

-
-
108-05-4
vinyl acetate

-
-
3663-82-9
2-hydroxymethyl-2,3-dihydrobenzo[1,4]dioxin

-
-
64179-44-8
acetic acid 2,3-dihydro-1,4-benzodioxin-2-ylmethyl ester

| Conditions | Yield |
|---|---|
| With N,N'-bismesityl-imidazol-2-ylidene In tetrahydrofuran at 20℃; for 0.25h; | 100% |
| 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene In tetrahydrofuran at 20℃; for 0.25h; | 100% |
| With P(MeNCH2CH2)3N In tetrahydrofuran for 2h; Ambient temperature; | 99% |
-
-
108-05-4
vinyl acetate

| Conditions | Yield |
|---|---|
| With Pseudomonas cepacia lipase In 1,4-dioxane at 30℃; for 120h; | 100% |
-
-
108-05-4
vinyl acetate

| Conditions | Yield |
|---|---|
| With Pseudomonas cepacia lipase In 1,4-dioxane at 30℃; for 48h; | 100% |
-
-
108-05-4
vinyl acetate

-
-
54445-64-6
(1R,2S)-(2-hydroxymethylcyclobutyl)methanol

-
-
123809-78-9
[(1S,2R)-2-(hydroxymethyl)cyclobutyl]methyl acetate

| Conditions | Yield |
|---|---|
| With pseudomonas fluorescens lipase at -2℃; for 13.75h; Acetylation; | 100% |
| With Amano lipase AK at -2℃; for 14h; Enzymatic reaction; optical yield given as %ee; | 99% |
-
-
108-05-4
vinyl acetate

-
-
187266-42-8, 327189-66-2
all-cis-5-(tert-butyldimethyl-silanyloxy)-cyclohexane-1,3-diol

-
-
327189-67-3
(1R,3S,5S)-1-acetoxy-3-hydroxy-5-(tert-butyldimethylsilanyloxy)-cyclohexane

| Conditions | Yield |
|---|---|
| With Lipase QL In ethyl acetate at 20℃; for 46h; Acetylation; | 100% |
| With Lipase QLM In ethyl acetate at 20℃; Enzymatic reaction; | 100% |
| With Lipase QL In ethyl acetate at 22℃; for 46h; | 99% |
| With amano lipase SP, from burkholderia cepacia In ethyl acetate at 20℃; Enzymatic reaction; | 2.356 g |
-
-
108-05-4
vinyl acetate

-
-
247025-10-1
3-Azido-3-deoxy-4-C-hydroxymethyl-1,2-O-isopropylidene-α-D-erythro-pentofuranose

-
-
551934-06-6
3-azido-5-O-acetyl-3-deoxy-4-C-hydroxymethyl-1,2-O-isopropylidene-α-D-erythro-ribofuranose

| Conditions | Yield |
|---|---|
| With Novozyme-435 In toluene at 25 - 28℃; Enzymatic reaction; regioselective reaction; | 100% |
| With Candida antarctica lipase In toluene at 25 - 28℃; | 98% |
-
-
108-05-4
vinyl acetate

-
-
534619-54-0
3,6-bis(3,4-dimethoxybenzoyl)-1,2,4,5-tetrazine

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 17h; | 100% |

| Conditions | Yield |
|---|---|
| With trimethyl orthoformate at 120℃; for 30h; | 100% |
| at 120℃; for 5h; Microwave irradiation; Sealed vial; | 99% |
| at 120℃; Sealed tube; Large scale; |

-
-
108-05-4
vinyl acetate

| Conditions | Yield |
|---|---|
| With lipase from Candida cylindracea at 25℃; for 6h; | 100% |

-
-
108-05-4
vinyl acetate

| Conditions | Yield |
|---|---|
| With lipase from Candida cylindracea at 25℃; for 6h; | 100% |
-
-
914803-06-8
(3R,5R,7S,9S,11S,13R)-3,13-Bis-benzyloxymethoxy-9-(tert-butyl-dimethyl-silanyloxy)-pentadecane-1,5,7,11,15-pentaol

-
-
108-05-4
vinyl acetate

-
-
914803-10-4
Acetic acid (3R,5S,7S,9S,11R,13R)-15-acetoxy-3,13-bis-benzyloxymethoxy-7-(tert-butyl-dimethyl-silanyloxy)-5,9,11-trihydroxy-pentadecyl ester

| Conditions | Yield |
|---|---|
| With lipase from Candida cylindracea at 25℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With Novozym 435(R) In acetonitrile at 30℃; for 16h; | 100% |
-
-
926035-11-2
(4R,5R)-4-acetoxy-7-chloro-5-methoxymethoxy-1-(p-toluenesulfonyl)-2,3,4,5-tetrahydro-1H-1-benzazepine

-
-
108-05-4
vinyl acetate

-
-
926035-13-4
(4R,5R)-7-chloro-4-hydroxy-5-methoxymethoxy-1-(p-toluenesulfonyl)-2,3,4,5-tetrahydro-1H-1-benzazepine

| Conditions | Yield |
|---|---|
| Stage #1: (4R,5R)-4-acetoxy-7-chloro-5-methoxymethoxy-1-(p-toluenesulfonyl)-2,3,4,5-tetrahydro-1H-1-benzazepine With sodium hydroxide In ethanol at 20℃; for 0.5h; Stage #2: vinyl acetate With Lipase QLM In acetonitrile at 65℃; for 1h; Stage #3: With sodium hydroxide In ethanol at 20℃; for 0.5h; Further stages.; | 100% |
-
-
108-05-4
vinyl acetate

-
-
3031-66-1
3-hexyn-2,5-diol

-
B
-
152694-53-6
Acetic acid (1R,4R)-4-acetoxy-1-methyl-pent-2-ynyl ester

-
C
-
152694-52-5
(2S,5S)-hex-3-yne-2,5-diol

| Conditions | Yield |
|---|---|
| With lipase AK at 32℃; for 16h; Enzymatic reaction; | A n/a B 100% C n/a |

Vinyl acetate Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 19 ,1979,p. 341.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 39 ,1986,p. 113.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 39 ,1986,p. 113.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory. Community Right-To-Know List. EPA Extremely Hazardous Substances List.
Vinyl acetate Standards and Recommendations
OSHA PEL: TWA 10 ppm; STEL 20 ppm
ACGIH TLV: 10 ppm, STEL: 15 ppm; Animal Carcinogen
DFG MAK: 10 ppm (35 mg/m3); Confirmed Animal Carcinogen with Unknown Relevance to Humans
NIOSH REL: (Vinyl Acetate) CL 15 mg/m3/15M
DOT Classification: 3; Label: Flammable Liquid
Vinyl acetate Analytical Methods
For occupational chemical analysis use OSHA: #51 or NIOSH: Vinyl Acetate, P&CAM 278.
Vinyl acetate Specification
The CAS registry number of Vinyl acetate is 108-05-4. The IUPAC name is ethenyl acetate. In addition, the molecular formula is C4H6O2. What's more, it is a colorless liquid with a pungent odor. And it is the precursor to polyvinyl acetate. It can be polymerized, either by itself to make polyvinyl acetate or with other monomers to prepare copolymers such as ethylene-vinyl acetate.
Physical properties about this chemical are: (1)ACD/LogP: 0.63; (2)ACD/LogD (pH 5.5): 0.629; (3)ACD/LogD (pH 7.4): 0.629; (4)ACD/BCF (pH 5.5): 1.77; (5)ACD/BCF (pH 7.4): 1.77; (6)ACD/KOC (pH 5.5): 52.382; (7)ACD/KOC (pH 7.4): 52.382; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 2; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.39; (12)Molar Refractivity: 22.081 cm3; (13)Molar Volume: 93.138 cm3; (14)Polarizability: 8.753 ×10-24cm3; (15)Surface Tension: 23.482 dyne/cm; (16)Density: 0.924 g/cm3; (17)Flash Point: °C; (18)Enthalpy of Vaporization: 31.389 kJ/mol; (19)Boiling Point: 72.499 °C at 760 mmHg; (20)Vapour Pressure: 118.484 mmHg at 25°C.
Preparation of Vinyl acetate: the major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst. The reaction temperature is 160-180 °C and the reaction pressure is below 1MPa. The equation is as follows: 2CH2=CH2 + 2CH3CO2H + O2 → 2CH2=CHOCOCH3. In addition, acetylene can react with acetic acid in the presence of zinc amalgam to give this chemical. The equation is as follows: CH≡CH + CH3CO2H → CH2=CHOCOCH3.
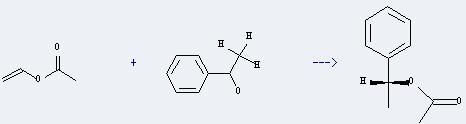
Uses of Vinyl acetate: it is mainly used for organic synthesis. In addition, it can react with 1-phenyl-ethanol to get (R)-1-acetoxy-1-phenyl-ethane. This reaction will need reagents Pseudomonas fluorescens Lipase, o-phenantroline and acetophenone, catalyst Rh2(OAc)4 and solvent CH2Cl2. The reaction time is 72 hours at reaction temperature of 20 °C. The yield is about 60%.
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable. And it has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. You can not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer). And in case of accident or if you feel unwell, seek medical advice immediately (show label where possible). What's more, you should keep away from sources of ignition and no smoking. In addition, you should not empty into drains. Keep container tightly closed after using it. Besides, you can take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(=O)OC=C
(2)InChI: InChI=1/C4H6O2/c1-3-6-4(2)5/h3H,1H2,2H3
(3)InChIKey: XTXRWKRVRITETP-UHFFFAOYAB
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC50 | inhalation | 6200ppm/4H (6200ppm) | National Technical Information Service. Vol. OTS0521596, | |
| guinea pig | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | LIVER: FATTY LIVER DEGERATION | American Industrial Hygiene Association Journal. Vol. 35, Pg. 21, 1974. |
| mouse | LC50 | inhalation | 1550ppm/4H (1550ppm) | E.I. Dupont de Nemours and Company, Technical Sheet. Vol. ES-3574, Pg. 1975, | |
| mouse | LD50 | oral | 1600mg/kg (1600mg/kg) | National Technical Information Service. Vol. OTS0521596, | |
| rabbit | LC50 | inhalation | 2500ppm/4H (2500ppm) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 621, 1986. | |
| rabbit | LD50 | skin | 2335mg/kg (2335mg/kg) | E.I. Dupont de Nemours and Company, Technical Sheet. Vol. ES-3574, Pg. 1975, | |
| rat | LC50 | inhalation | 11400mg/m3/4H (11400mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 25(11), Pg. 57, 1981. | |
| rat | LD50 | oral | 2900mg/kg (2900mg/kg) | National Technical Information Service. Vol. OTS0521596, |
Related Products
- VINYL 2-(BUTYLMERCAPTOETHYL) ETHER
- Vinyl acetate
- Vinyl acetate-crotonic acid-vinyl neodecanoate terpolymer
- VINYL AZIDE
- Vinyl Benzoate
- Vinyl bromide
- Vinyl butyrate
- Vinyl carbamate
- Vinyl chloride
- Vinyl chloroacetate
- 1080622-86-1
- 108063-02-1
- 1080644-24-1
- 108078-14-4
- 1080-79-1
- 108-08-7
- 108097-02-5
- 108097-04-7
- 108097-98-9
- 108-09-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View