SICHUAN TONGSHENG AMINOACID CO.LTD
Sichuan Tongsheng is the most strongest manufacturer and exporter of amino acids and their derivatives in China, we have the best quality and price. Guarantee high quality, competive price and reliable service. We fully compliance with ISO9001:2008,
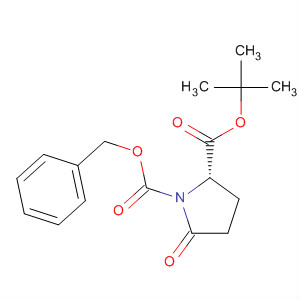
Cas:113400-36-5
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:113400-36-5
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryLeader Biochemical Group
About Product Details
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $1.0 / 5.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $1.0 / 10.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
Product Name: Boc-Pyr-Obzl CAS#: 113400-36-5 Synonyms: Boc-Pyroglutamicacid. benzyl ester Appearance: white powder Storage:Store in cool and dry place, away from sun light. Package: 25kgs/drum Application:Flav
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:113400-36-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $89.0 / 100.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
BOC-L-PYROGLUTAMIC ACID BENZYL ESTER CAS: 113400-36-5 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high qu
Cas:113400-36-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:113400-36-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Hangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:113400-36-5
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:113400-36-5
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:113400-36-5
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
Benzyl N-Boc glutamateAppearance:White fine powder Storage:Kept in a cool,dry and ventilated place Package:according to customers' requirements Application:Meets the requirements Transportation:By air(EMS or EUB or FedEx or TNT ect...) or by sea(FOB
SHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Cas:113400-36-5
Min.Order:100 Gram
FOB Price: $100.0 / 2000.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 6 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $1000.0
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:113400-36-5
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:113400-36-5
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Wuhu Nuowei Chemistry Technologies Co., Ltd.
Wuhu Nuovo Chemical Technology Co., Ltd. was established in August 2014, mainly engaged in the development, production and sales of ionic liquids, ribose, nucleosides, nucleotides and related chemicals; Products are mainly used in new energy, new ma
Cas:113400-36-5
Min.Order:100 Gram
Negotiable
Type:Manufacturers
inquirySAGECHEM LIMITED
SAGECHEM is a chemical R&D, manufacturing and distribution company in China since 2009, including pharmaceutical intermediates, agrochemical, dyestuff intermediates, organosilicone, API and etc. We also offer a full range of services in custom synthe
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Win-Win chemical Co.Ltd
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryResearch Peptide Biotechnology Co., Ltd.
High purity, high success rate, short cycle and moderate priceAppearance:White powder solid Storage:Negative 20 degrees Celsius Package:5mg, 10mg 100mg, 1gram Application:Applied to various scientific research
Cas:113400-36-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
94885-52-6
benzyl (L)-pyroglutamate

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; | 98% |
| With dmap; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 3h; | 90% |
| With dmap; triethylamine In dichloromethane at 0 - 23℃; for 17h; Inert atmosphere; | 90% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4561-10-8
L-glutamic acid dibenzyl ester hydrochloride

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In toluene at 30 - 95℃; for 9h; Solvent; Temperature; Concentration; | 94.6% |
-
-
98-79-3
L-Pyroglutamic acid

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| 94% | |
| Multi-step reaction with 2 steps 1: Et3N / tetrahydrofuran / 120 h / Heating 2: 9.0 g / Et3N; 4-(dimethylamino)pyridine / CH2Cl2 / 384 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / TEA / acetone 2: 73 percent / DMAP / acetonitrile View Scheme |
-
-
135347-23-8
(2S,4S)-4-((R)-3-Methyl-1,1-dioxo-2,3-dihydro-1H-1λ6-benzo[d]isothiazol-3-yl)-5-oxo-pyrrolidine-1,2-dicarboxylic acid 2-benzyl ester 1-tert-butyl ester

-
A
-
34989-82-7
3-methylbenzo[d]isothiazole-1,1-dioxide

-
B
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran 1.) -78 deg C, 30 min, 2.) -78 deg C -> room temperature, 2 h; | A 40% B 38% |
-
-
75-16-1
methylmagnesium bromide

-
A
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
B
-
113400-46-7
benzyl (2S)-2-[(tert-butoxycarbonyl)amino]-5-oxohexanoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at -78℃; for 0.75h; Yield given. Yields of byproduct given; |


-
A
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
B
-
113400-46-7
benzyl (2S)-2-[(tert-butoxycarbonyl)amino]-5-oxohexanoate

| Conditions | Yield |
|---|---|
| With methylmagnesium bromide In tetrahydrofuran; toluene at -78℃; for 0.75h; Yield given. Yields of byproduct given; |
-
-
100-44-7
benzyl chloride

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Et3N / tetrahydrofuran / 120 h / Heating 2: 9.0 g / Et3N; 4-(dimethylamino)pyridine / CH2Cl2 / 384 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine / tetrahydrofuran / 120 h / 70 °C 2: triethylamine; dmap / dichloromethane / 16 h / 20 °C / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine / acetone / 24 °C / Reflux; Inert atmosphere 2: dmap / dichloromethane / Inert atmosphere View Scheme |
-
-
100-44-7
benzyl chloride

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / TEA / acetone 2: 73 percent / DMAP / acetonitrile View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N-diisopropylethylamine / CH2Cl2 / 5 h / Heating 2: 37.5 g / 4-dimethylaminopyridine / acetonitrile / 3 h / 25 °C View Scheme |
-
-
100-39-0
benzyl bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 88 percent / Et3N / acetone / 24 h / 20 °C 2: 90 percent / DIEA; DMAP / dimethylformamide / 3 h / 20 °C View Scheme |
-
-
98-79-3
L-Pyroglutamic acid

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 88 percent / Et3N / acetone / 24 h / 20 °C 2: 90 percent / DIEA; DMAP / dimethylformamide / 3 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / SOCl2; DMF / 20 °C 2: 98 percent / dimethylaminopyridine / CH2Cl2 / 0 - 20 °C View Scheme |
-
-
100-51-6
benzyl alcohol

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / SOCl2; DMF / 20 °C 2: 98 percent / dimethylaminopyridine / CH2Cl2 / 0 - 20 °C View Scheme |
-
-
100-51-6
benzyl alcohol

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / SOCl2 / 0 - 20 °C 2: Et3N; DMAP View Scheme |
-
-
100-39-0
benzyl bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: DIEA / CH2Cl2 / Heating 2: 9.56 g / DMAP; Et3N / acetonitrile / 20 °C View Scheme |
-
-
30924-93-7
Boc-Glu-OBn

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 4-methylmorpholine / CH2Cl2 / 0.25 h / 0 °C 2: tetrahydrofuran; toluene / 0.75 h / -78 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 4-methylmorpholine / CH2Cl2 / 0.25 h / 0 °C 2: methylmagnesium bromide / tetrahydrofuran; toluene / 0.75 h / -78 °C View Scheme |
-
-
100-44-7
benzyl chloride

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NEt3 / tetrahydrofuran / 120 h / Heating 2: NEt3 / CH2Cl2 View Scheme |
-
-
100-44-7
benzyl chloride

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / triethylamine / acetone / 168 h / Heating 2: 75 percent / DMAP / acetonitrile / 1.) 0 deg C, 2 h, 2.) r.t., overnight View Scheme |
-
-
34619-03-9
tert-butyldicarbonate

-
-
94885-52-6
benzyl (L)-pyroglutamate

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 4h; |
-
-
100-51-6
benzyl alcohol

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride / N,N-dimethyl-formamide / 0 - 20 °C 2: dmap / dichloromethane / 0 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: thionyl chloride / 16 h / 0 - 23 °C / Inert atmosphere 2: dmap; triethylamine / dichloromethane / 17 h / 0 - 23 °C / Inert atmosphere View Scheme |
-
-
100-44-7
benzyl chloride

-
-
98-79-3
L-Pyroglutamic acid

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; potassium iodide / toluene / 80 °C 2: 4-pyrrolidin-1-ylpyridine / 50 °C View Scheme |
-
-
100-39-0
benzyl bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / acetone / 16 h / Reflux 2: dmap; triethylamine / dichloromethane / 16 h / 50 °C / Inert atmosphere View Scheme |
-
-
1774-47-6
trimethylsulfoxonium iodide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsulfoxonium iodide With potassium tert-butylate In tetrahydrofuran; dimethyl sulfoxide at 20℃; for 1h; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran; dimethyl sulfoxide at -10℃; | 100% |
| With potassium carbonate In dimethyl sulfoxide at 50℃; for 23h; Temperature; Reagent/catalyst; Solvent; | 96% |
| Stage #1: trimethylsulfoxonium iodide With potassium tert-butylate In tetrahydrofuran; dimethyl sulfoxide at 20℃; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran; dimethyl sulfoxide at -12℃; | |
| Stage #1: trimethylsulfoxonium iodide With potassium tert-butylate; dimethyl sulfoxide In tetrahydrofuran at 25℃; for 1h; Large scale; Industrial scale; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran at -12℃; Large scale; Industrial scale; | |
| Stage #1: trimethylsulfoxonium iodide With potassium tert-butylate In tetrahydrofuran; dimethyl sulfoxide at 25℃; for 2h; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran; dimethyl sulfoxide at 0 - 10℃; for 8h; |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
331949-29-2
benzyl (2S)-N-tert-butoxycarbonyl-5-hydroxypyrrolidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran at -78℃; for 2h; | 99% |
| With lithium triethylborohydride In tetrahydrofuran at -78℃; for 0.5h; | 95% |
| With lithium triethylborohydride In tetrahydrofuran at -78℃; for 2h; | 88% |
| With diisobutylaluminium hydride In tetrahydrofuran at -78℃; | 70% |
| With lithium triethylborohydride In tetrahydrofuran at -78℃; |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
53100-44-0
L-N-t-butoxycarbonylpyroglutamic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate for 120h; Ambient temperature; | 98% |
| With hydrogen; 10percent Pd/C In ethyl acetate under 2280.15 Torr; for 1h; | 98% |
| With hydrogen; palladium on activated charcoal In methanol for 6h; | 93% |
-
-
67-56-1
methanol

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
331949-31-6
benzyl (2S)-N-tert-butoxycarbonyl-5-methoxypyrrolidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With diisobutylaluminium hydride In tetrahydrofuran at -78℃; Stage #2: methanol With toluene-4-sulfonic acid In tetrahydrofuran at 20℃; Further stages.; | 95% |
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium triethylborohydride In tetrahydrofuran at -78℃; Inert atmosphere; Stage #2: With dihydrogen peroxide In tetrahydrofuran; water at -78 - 20℃; Inert atmosphere; Stage #3: methanol With toluene-4-sulfonic acid In water at 20℃; Inert atmosphere; chemoselective reaction; | 86% |
-
-
399-94-0
2-bromo-1,4-difluorobenzene

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-1,4-difluorobenzene With isopropylmagnesium chloride In tetrahydrofuran at 0℃; for 2h; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran at 0℃; for 4h; | 93% |
-
-
32359-01-6
hexenylmagnesium bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
113400-48-9
(S)-2-tert-Butoxycarbonylamino-5-oxo-undec-6-ynoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 2h; | 92% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
91229-97-9
(S)-2-[N-(tert-butoxycarbonyl)amino]-5-hydroxypentanoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; potassium dihydrogenphosphate In methanol; water at 0℃; for 1.5h; Reduction; | 92% |
| With sodium tetrahydroborate; water In tetrahydrofuran at 0 - 23℃; for 2h; Inert atmosphere; | 49% |
| With sodium tetrahydroborate; potassium dihydrogenphosphate In methanol; water at 0℃; for 2h; | 12% |
-
-
917-54-4
methyllithium

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
113400-46-7
benzyl (2S)-2-[(tert-butoxycarbonyl)amino]-5-oxohexanoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -78 - 20℃; for 2h; Inert atmosphere; | 91% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
91229-91-3
tert-butyl (S)-N-tert-butoxycarbonylpyroglutamate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With hydrogen; palladium on activated charcoal In ethyl acetate Stage #2: di-tert-butyl dicarbonate With dmap; TEA In acetonitrile Further stages.; | 83% |
| With dmap; hydrogen; triethylamine 2.) CH3CN; Multistep reaction; |
-
-
1826-67-1
vinyl magnesium bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
113400-37-6
2-tert-butoxycarbonylamino-5-oxo-hept-6-enoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 2h; | 82% |
-
-
917-64-6
methyl magnesium iodide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
113400-46-7
benzyl (2S)-2-[(tert-butoxycarbonyl)amino]-5-oxohexanoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 2h; | 78% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
591-51-5
phenyllithium

-
-
148626-26-0
(S)-2-tert-Butoxycarbonylamino-5-oxo-5-phenyl-pentanoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 2h; | 77% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
756-79-6
dimethyl methane phosphonate

-
-
270585-72-3
(2S)-α-benzyl 2-N-(tert-butyloxycarbonyl)amino-5-oxo-6-(dimethylphosphonyl)hexanoate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In toluene at -78℃; for 0.333333h; Metallation; Stage #2: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In toluene at -78 - 20℃; Addition; Further stages.; | 74% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
208519-99-7
(S)-2-tert-Butoxycarbonylamino-6-diazo-5-oxo-hexanoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -105 - -100℃; for 0.166667h; | 71% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
1730-25-2
allylmagnesium bromide

-
-
113400-47-8
(S)-2-tert-Butoxycarbonylamino-5-oxo-oct-7-enoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 2h; | 70% |
-
-
100-39-0
benzyl bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
1243255-28-8
2-benzyl 1-(tert-butyl) (2S)-4-benzyl-5-oxo-1,2-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1.08333h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran at -78℃; for 16.05h; Inert atmosphere; stereoselective reaction; | 69% |
-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With cesium fluoride In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; | 66% |
-
-
33797-51-2
eschenmoser's salt

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
159829-96-6
(S)-4,4-Bis-dimethylaminomethyl-5-oxo-pyrrolidine-1,2-dicarboxylic acid 2-benzyl ester 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.666667h; | 65% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -80℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at -80 - 20℃; | 64.9% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
94885-52-6
benzyl (L)-pyroglutamate

| Conditions | Yield |
|---|---|
| With trimethylaluminum In dichloromethane Ambient temperature; | 64% |
-
-
7103-09-5
4-but-1-enylmagnesium bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
1282532-98-2
(S)-2-tert-butoxycarbonylamino-5-oxo-non-8-enoic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-but-1-enylmagnesium bromide; benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran at -40℃; for 3h; Stage #2: With ammonium chloride In tetrahydrofuran; water | 62% |
| Stage #1: 4-but-1-enylmagnesium bromide; benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate In tetrahydrofuran at -40℃; for 3h; Stage #2: With water; ammonium chloride In tetrahydrofuran | 62% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
79-22-1
methyl chloroformate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 2h; Stage #2: methyl chloroformate In tetrahydrofuran at -78℃; for 3h; | 60% |
-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
870-63-3
prenyl bromide

-
-
1243255-29-9
2-benzyl 1-(tert-butyl) (2S)-4-(3-methyl-2-butenyl)-5-oxo-1,2-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1.08333h; Inert atmosphere; Stage #2: prenyl bromide In tetrahydrofuran at -78℃; for 16.05h; Inert atmosphere; stereoselective reaction; | 60% |
-
-
96090-12-9, 124854-99-5
1-(4-bromophenyl)-1-propen-3-yl bromide

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Activation; Stage #2: 1-(4-bromophenyl)-1-propen-3-yl bromide In tetrahydrofuran at -78℃; for 2h; Alkylation; | A 58% B n/a |
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Substitution; Stage #2: 1-(4-bromophenyl)-1-propen-3-yl bromide In tetrahydrofuran at -78℃; for 2h; Alkylation; | A 58% B n/a |

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
116857-24-0
benzyl (2S,4R)-1-tert-butoxycarbonyl-4-hydroxy-5-oxopyrrolidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Stage #2: With N-(benzenesulfonyl)-3-phenyloxaziridine In tetrahydrofuran at -78℃; for 0.75h; Stage #3: With camphor-10-sulfonic acid; water In tetrahydrofuran at -78 - 20℃; stereoselective reaction; | 55% |
| With 2-toluenesulfonyl-3-phenyloxazolidine; lithium hexamethyldisilazane 1.) THF, -78 deg C; Yield given. Multistep reaction; |
-
-
4224-69-5
methyl 2-(bromomethyl)propenoate

-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 2h; Yields of byproduct given; | A 54% B n/a |
| With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 2h; Yield given; Yields of byproduct given. Title compound not separated from byproducts; |


-
-
113400-36-5
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate

-
-
4392-24-9
Cinnamyl bromide

-
-
220424-73-7
Boc-4(R)-(3-phenylpropyl)proline

| Conditions | Yield |
|---|---|
| With ammonium chloride In tetrahydrofuran | 54% |

Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View