Chemvon Biotechnology Co. Ltd.
Chemvon Biotechnology is one of the leading high-technology manufacturer in the field of pharmaceutical and fine chemical industry. From origins as a research group in technology service, chemvon has progressed into an integrated company with a k
Cas:120279-96-1
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Our company engages in Sodium Tripolyphosphate (STPP) and Sodium Hexametabphosphate (SHMP) production; development of noble metal catalysts, synthesis of electronic chemical materials and general chemicals Imp&Exp trading business. The company
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $1000.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $1.0 / 10.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:120279-96-1
Min.Order:10 Gram
FOB Price: $146.0 / 176.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
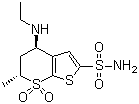
[English Name] Dorzolamide [CAS No.] 120279-96-1 [Mol. Formula ] C10H16N2O4S3 Appearance:White powder Storage:Store in cool and dry place, away from sun light. Package:drum Application:Eye Treatment Drug; Miotic, Antiglaucoma Transportation:B
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:120279-96-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Dorzolamide CAS:120279-96-1 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediate
Cas:120279-96-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Changchun Artel lmport and Export trade company
Superiority We can customize and synthesize products that other suppliers may not be able to provide. Advantage cof, mof ligand manufacturer best service, company and transport
Cas:120279-96-1
Min.Order:100 Kilogram
Negotiable
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Afine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $1.0 / 100000.0
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:120279-96-1
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Chemlyte Solutions
Chemlyte Solutions believe that customers and suppliers deserve much more than what traditional distributors can offer. To grow in today s fast-paced and increasingly competitive market it is essential to be able to quickly adapt to market forces eff
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:120279-96-1
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providing h
Zibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Jiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Hangzhou Think Chemical Co. Ltd
HANGZHOU THINK CHEMICAL CO., LTD. (THINKCHEM) is an integrative corporation of trade, research and contract manufacture. With about ten years of business experiences on the marketing & distribution, thinkchem specializes in exp
Cas:120279-96-1
Min.Order:0 Metric Ton
Negotiable
Type:Other
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryTAIZHOU ZHENYU BIOTECHNOLOGY CO., LTD
Zhenyu biotech exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, zhenyu biotech is your best choice. pls contact with us freely for getting detailed
Cas:120279-96-1
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:120279-96-1
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Suzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:Active Pharmaceutical I
Golden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
Bluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Kono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
Synthetic route
-
-
147200-03-1
(4S,6S)‑4‑acetamido‑5,6‑dihydro‑6‑methyl‑4H‑thieno[2,3‑b]thiopyran‑2‑sulfonamide‑7,7‑dioxide

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With zinc(II) tetrahydroborate In methanol at 90℃; for 3h; Temperature; Solvent; Green chemistry; | 90% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In methanol for 72h; Heating / reflux; | 66% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; sulfuric acid 1) THF, r.t.; Yield given. Multistep reaction; |
-
-
147086-79-1
(S)-5,6-dihydro-6-methylthieno<2,3-b>thiopyran-4-one

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 2: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 3: 95 percent / pyridine / tetrahydrofuran / 48 h / 25 - 30 °C 4: 89.6 percent / H2SO4 / 20 - 25 °C 5: chlorosulfonic acid / 12 h / 50 °C 6: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 7: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 7 steps 1: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 2: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 3: H2SO4 / -5 °C 4: H2SO4 / -5 °C 5: chlorosulfonic acid / 12 h / 50 °C 6: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 7: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 6 steps 1: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 2: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 3: 1) conc. H2SO4 / 1) THF, 48 h, r.t., 2) overnight 4: chlorosulfonic acid / 12 h / 50 °C 5: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 6: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
147086-80-4
5,6-dihydro-(R)-4-hydroxy-(S)-6-methyl-4H-thieno<2,3-b>thiopyran

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 2: 95 percent / pyridine / tetrahydrofuran / 48 h / 25 - 30 °C 3: 89.6 percent / H2SO4 / 20 - 25 °C 4: chlorosulfonic acid / 12 h / 50 °C 5: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 6: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 6 steps 1: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 2: H2SO4 / -5 °C 3: H2SO4 / -5 °C 4: chlorosulfonic acid / 12 h / 50 °C 5: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 6: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 5 steps 1: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 2: 1) conc. H2SO4 / 1) THF, 48 h, r.t., 2) overnight 3: chlorosulfonic acid / 12 h / 50 °C 4: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 5: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
133359-80-5
(S)-3-(thien-2-ylthio)butyric acid

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: trifluoroacetic anhydride / toluene / 1 h / 25 °C 2: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 3: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 4: 95 percent / pyridine / tetrahydrofuran / 48 h / 25 - 30 °C 5: 89.6 percent / H2SO4 / 20 - 25 °C 6: chlorosulfonic acid / 12 h / 50 °C 7: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 8: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 8 steps 1: trifluoroacetic anhydride / toluene / 1 h / 25 °C 2: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 3: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 4: H2SO4 / -5 °C 5: H2SO4 / -5 °C 6: chlorosulfonic acid / 12 h / 50 °C 7: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 8: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 7 steps 1: trifluoroacetic anhydride / toluene / 1 h / 25 °C 2: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 3: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 4: 1) conc. H2SO4 / 1) THF, 48 h, r.t., 2) overnight 5: chlorosulfonic acid / 12 h / 50 °C 6: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 7: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
147086-84-8
(S)-6-Methyl-6H-thieno[2,3-b]thiopyran 7,7-dioxide

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2SO4 / -5 °C 2: chlorosulfonic acid / 12 h / 50 °C 3: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 4: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
133359-79-2
methyl (S)-3-(thiophen-2-ylthio)butyrate

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 45 g / 12 N HCl / H2O / 3 h / Heating 2: trifluoroacetic anhydride / toluene / 1 h / 25 °C 3: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 4: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 5: 95 percent / pyridine / tetrahydrofuran / 48 h / 25 - 30 °C 6: 89.6 percent / H2SO4 / 20 - 25 °C 7: chlorosulfonic acid / 12 h / 50 °C 8: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 9: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 9 steps 1: 45 g / 12 N HCl / H2O / 3 h / Heating 2: trifluoroacetic anhydride / toluene / 1 h / 25 °C 3: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 4: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 5: H2SO4 / -5 °C 6: H2SO4 / -5 °C 7: chlorosulfonic acid / 12 h / 50 °C 8: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 9: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 8 steps 1: 45 g / 12 N HCl / H2O / 3 h / Heating 2: trifluoroacetic anhydride / toluene / 1 h / 25 °C 3: LiAlH4 / tetrahydrofuran; toluene / 0 - 5 °C 4: 24 g / sodium tungstate dihydrate, 30 percent hydrogen peroxide / H2O; ethyl acetate; toluene / 1) 0 - 15 deg C, 30 min, 2) 25 deg C, 2 h 5: 1) conc. H2SO4 / 1) THF, 48 h, r.t., 2) overnight 6: chlorosulfonic acid / 12 h / 50 °C 7: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 8: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
1034290-08-8
5,6-dihydro-(R,S)-4-hydroxy-(S)-6-methyl-4H-thieno<2,3-b>thiopyran 7,7-dioxide

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 95 percent / pyridine / tetrahydrofuran / 48 h / 25 - 30 °C 2: 89.6 percent / H2SO4 / 20 - 25 °C 3: chlorosulfonic acid / 12 h / 50 °C 4: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 5: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 5 steps 1: H2SO4 / -5 °C 2: H2SO4 / -5 °C 3: chlorosulfonic acid / 12 h / 50 °C 4: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 5: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme | |
| Multi-step reaction with 4 steps 1: 1) conc. H2SO4 / 1) THF, 48 h, r.t., 2) overnight 2: chlorosulfonic acid / 12 h / 50 °C 3: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 4: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 2: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: chlorosulfonic acid / 12 h / 50 °C 2: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 3: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 89.6 percent / H2SO4 / 20 - 25 °C 2: chlorosulfonic acid / 12 h / 50 °C 3: conc. NH4OH / tetrahydrofuran / 1 h / 0 - 5 °C 4: 1) borane-dimethyl sulfide, 2) H2SO4 / 1) THF, r.t. View Scheme |
-
-
120279-96-1
dorzolamide

-
-
130693-82-2
dorzolamide hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate pH=1; | 95% |
| With hydrogenchloride In water; dimethyl sulfoxide at 5 - 10℃; for 3h; pH=1.0; |
-
-
6217-54-5
docosahexaenoic acid

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: docosahexaenoic acid With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 95% |
-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With trimethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 16h; Inert atmosphere; | 95% |
-
-
(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[ (2S)-2-{[ (2S)-2-{[(2S)-2-{[(2S)-2-[(tert-butyldiphenylsilyl)oxy]propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoic acid

-
-
120279-96-1
dorzolamide

-
-
(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-[(1S)-1-({[(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1λ6-thieno[2,3-b]thiopyran-6-yl]sulfonyl}carbamoyl)ethoxy]-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl (2S)-2-[(tert-butyldiphenylsilyl)oxy]propanoate

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[ (2S)-2-{[ (2S)-2-{[(2S)-2-{[(2S)-2-[(tert-butyldiphenylsilyl)oxy]propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 84% |
-
-
(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-[(tert butyldiphenylsilyl)oxy]propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoic acid

-
-
120279-96-1
dorzolamide

-
-
(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-[(1S)-1-({[(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1λ6-thieno[2,3-b]thiopyran-6-yl]sulfonyl}carbamoyl)ethoxy]-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl (2S)-2-[(tert-butyldiphenylsilyl)oxy]propanoate

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; Inert atmosphere; Stage #2: (2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-[(tert butyldiphenylsilyl)oxy]propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 84% |
-
-
4530-20-5
BOC-glycine

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With triethylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: BOC-glycine With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 82% |
-
-
120279-96-1
dorzolamide

-
-
58479-61-1
tert-butylchlorodiphenylsilane

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 30℃; for 2h; | 76% |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 3h; Inert atmosphere; | 49% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 4h; Inert atmosphere; | 74% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With triethylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: {[(acetyloxy)acetyl](methyl)amino}acetic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 72.7% |

-
-
120279-96-1
dorzolamide

-
-
(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-[(1S)-1-({[(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1λ6-thieno[2,3-b]thiopyran-6-yl]sulfonyl}carbamoyl)ethoxy]-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl (2S)-2-[(tert-butyldiphenylsilyl)oxy]propanoate

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-[(tert butyldiphenylsilyl)oxy]propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 72% |
-
-
120279-96-1
dorzolamide

-
-
125941-75-5
(S)-2-[(1,1-dimethylethyl)diphenylsilyl]oxypropanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-[(1,1-dimethylethyl)diphenylsilyl]oxypropanoic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 68% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-(tert-butyldiphenylsilanyloxy)propionic acid (S)-1-((S)-1-carboxyethoxycarbonyl)ethyl ester With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 68% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-(tert-butyl-diphenyl-silanyloxy)-propionic acid (S)-1-[(S)-1-((S)-1-carboxy-ethoxycarbonyl)-ethoxycarbonyl]-ethyl ester With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 65% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 65% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-(tert-butyldiphenylsilanyloxy)propionic acid (S)-1-{(S)-1-[(S)-1-((S)-1-carboxyethoxycarbonyl)ethoxycarbonyl]ethoxycarbonyl}ethyl ester With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 63% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-(tert-butyl-diphenyl-silanyloxy)-propionic acid (S)-1-carboxy-ethyl ester With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 60% |
-
-
120279-96-1
dorzolamide

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: Acetoxyacetyl chloride With dmap In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 59.6% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: carbonochloridic acid, chloromethyl ester In dichloromethane at 0 - 30℃; for 1h; Temperature; Inert atmosphere; | 57% |
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 25 - 30℃; for 0.5h; Stage #2: carbonochloridic acid, chloromethyl ester In dichloromethane at 0 - 5℃; for 1h; |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 4h; Inert atmosphere; | 50% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 48% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 2h; Inert atmosphere; | 45.1% |

-
-
120279-96-1
dorzolamide

-
-
(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-{[(2S)-1-[(1S)-1-({[(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1λ6-thieno[2,3-b]thiopyran-6-yl]sulfonyl}carbamoyl)ethoxy]-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl (2S)-2-(acetyloxy)propanoate

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-(acetyloxy)propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy} propanoic acid With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 2h; Inert atmosphere; | 42% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: octadecanoic acid (S)-1-{(S)-1-[(S)-1-((S)-1-carboxyethoxycarbonyl)ethoxy carbonyl]ethoxycarbonyl}ethyl ester With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 41% |
-
-
108-24-7
acetic anhydride

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With triethylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: acetic anhydride In dichloromethane at 0 - 30℃; for 1h; Inert atmosphere; | 40% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-acetoxypropionyloxy)propionyloxy]propionyloxy}propionyloxy)propionic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 1h; | 38% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2S)-2-{[(2S)-2-[(4-{[(3Z)-3-[(4-{[2-(diethylamino)ethyl]carbamoyl}-3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-2-oxo-2,3-dihydro-1H-indol-5-yl]oxy}-4-oxobutanoyl)oxy]propanoyl]oxy}propanoic acid With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 2h; | 38% |

-
-
120279-96-1
dorzolamide

-
-
(3Z)-3-[(4-{[2-(diethylamino)ethyl]carbamoyl}-3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-2-oxo-2,3-dihydro-1H-indol-5-yl (2S)-1-{[(2S)-1-{[(2S)-1-[(1S)-1-({[(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1λ6-thieno[2,3-b]thiopyran-6-yl]sulfonyl}carbamoyl)ethoxy]-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl]oxy}-1-oxopropan-2-yl butanedioate

| Conditions | Yield |
|---|---|
| Stage #1: dorzolamide With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: succinic acid (S)-1-{(S)-1-[(S)-1-((S)-1-carboxyethoxycarbonyl)ethoxycarbonyl]ethoxycarbonyl}ethyl ester 3-[1-[4-(2-diethylaminoethylcarbamoyl)-3,5-dimethyl-1H-pyrrol-2-yl]-meth-(Z)-ylidene]-2-oxo-2,3-dihydro-1H-indol-5-yl ester With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 2h; | 38% |

-
-
120279-96-1
dorzolamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 30℃; for 16h; | 38% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View