Hunan Russell Chemicals Technology Co.,Ltd
low price and high purityAppearance:solid or liquid Storage:in sealed air resistant place Package:As customer require Application:Pharma;Industry;Agricultural Transportation:by sea or by airplane Port:any port in China
Cas:22128-62-7
Min.Order:0
Negotiable
Type:Trading Company
inquirySynthetic route
-
-
79-22-1
methyl chloroformate

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With chlorine at 65 - 88℃; for 4h; Yields of byproduct given; | A n/a B 22% |
| With chlorine at 65 - 88℃; for 4h; Yield given. Yields of byproduct given; | |
| With 2,2'-azobis(isobutyronitrile); chlorine at 70℃; for 9.5h; | A 1.94 %Chromat. B 68.5 %Chromat. |
-
-
107-31-3
Methyl formate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| bei gemaessigter Chlorierung am Licht; |
-
-
107-31-3
Methyl formate

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
C
-
503-38-8
trichloromethyl chloroformate

| Conditions | Yield |
|---|---|
| Bei der Chlorierung im Licht; | |
| With oxygen; chlorine at 22.85℃; Kinetics; Further Variations:; Reagents; |

-
-
56-23-5
tetrachloromethane

-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| at 25℃; Kinetics; Photochlorierung; |
-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| bei gemaessigter Chlorierung am Licht; | |
| With chlorine Ambient temperature; Irradiation; | |
| With sulfuryl dichloride; Perbenzoic acid Heating; |

| Conditions | Yield |
|---|---|
| benzyltri(n-butyl)ammonium chloride at 0 - 20℃; for 0.5h; |
-
-
7782-50-5
chlorine

-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| at 23.5 - 27℃; durch UV-Licht (λ:435.8 nm) initiierten Reaktion in der Dampfphase; |
-
-
107-31-3
Methyl formate

-
-
7782-50-5
chlorine

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| im diffusen Licht; |

-
-
123-75-1
pyrrolidine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
93765-67-4
N-(chloromethyloxycarbonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 2h; Substitution; | 100% |
| With pyridine Yield given; |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
100-61-8
N-methylaniline

-
-
186353-05-9
[N-methyl-N-phenyl]carbamic acid chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 100% |
| With triethylamine In dichloromethane at 0 - 5℃; for 4h; | 73% |
-
-
44897-15-6
sarcosine diethylamide

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
934548-22-8
N-(chloromethyloxycarbonyl)sarcosine diethylamide

| Conditions | Yield |
|---|---|
| In dichloromethane at -10 - 20℃; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; for 0.75h; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane; water at 20℃; for 2h; | 100% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
108-98-5
thiophenol

-
-
133217-39-7
Thiocarbonic acid O-chloromethyl ester S-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether 1) 0 to 5 deg C, 30 min, 2) RT, 16h; | 99% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
158991-23-2
PD 154075

-
-
247017-85-2
3-[(R)-2-(Benzofuran-2-ylmethoxycarbonylamino)-2-((S)-1-phenyl-ethylcarbamoyl)-propyl]-indole-1-carboxylic acid chloromethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: PD 154075 With potassium hexamethylsilazane In tetrahydrofuran at -78℃; Metallation; Stage #2: carbonochloridic acid, chloromethyl ester In tetrahydrofuran at -78℃; Acylation; | 99% |
-
-
112-35-6
triethylene glucol monomethyl ether

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
209551-63-3
chloromethyl (2-(2-(2-methoxyethoxy)ethoxy)ethyl) carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 22h; Inert atmosphere; | 99% |
| With pyridine In dichloromethane at 0 - 20℃; | 99% |
| With pyridine In dichloromethane | |
| With pyridine In dichloromethane at 20℃; | |
| With pyridine In dichloromethane at -78℃; for 3h; |
-
-
110-91-8
morpholine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
93765-68-5
chloromethyl morpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 0.5h; | 99% |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.58333h; |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 99% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In 1,2-dichloro-ethane; N,N-dimethyl-formamide at 60℃; for 1.08333h; | 99% |
-
-
107-03-9
1-thiopropane

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tert-butyl methyl ether at 5 - 20℃; | 99% |
| With N-ethyl-N,N-diisopropylamine In tert-butyl methyl ether at 5 - 20℃; | 29 g |
-
-
32399-12-5
2-(N-methylamino)-3-hydroxymethylpyridine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 5℃; for 0.0833333h; | 99% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -10℃; for 1h; |
-
-
1557084-43-1
3-(2-chloro-4-morpholinopyrido[3,2-d]pyrimidin-7-yl)aniline

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
1557084-82-8
chloromethyl (3-(2-chloro-4-morpholinopyrido[3,2-d]pyrimidin-7-yl)phenyl)carbamate

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In chloroform at 20℃; Cooling with ice; | 98.4% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
67-63-0
isopropyl alcohol

-
-
35180-01-9
chloromethyl isopropyl carbonate

| Conditions | Yield |
|---|---|
| With triethylamine at 0℃; | 98% |
| With pyridine In diethyl ether; isopropyl alcohol at 10℃; for 18h; Cooling; | 95% |
| With pyridine In diethyl ether at 0 - 20℃; | 92% |
-
-
111-87-5
octanol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
920967-14-2
chloromethyl-1-octyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 98% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane | |
| With triethylamine In dichloromethane at 0 - 20℃; for 21h; |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at -40 - 20℃; for 0.333333h; | 98% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
109-89-7
diethylamine

-
-
133217-92-2
chloromethyl N,N-diethyl carbamate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 0.5h; | 97% |
| In hexane at -10 - -5℃; for 2.25h; | 92% |
| With triethylamine In diethyl ether 1) 0 to 5 deg C, 30 min, 2) RT, 16h; | 71% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
111-27-3
hexan-1-ol

-
-
663597-51-1
chloromethyl hexyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 25℃; | 97% |
| With pyridine In dichloromethane at 0 - 20℃; for 20h; | 63% |
-
-
90-15-3
α-naphthol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
132905-86-3
Carbonic acid chloromethyl ester naphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 5℃; for 1.66667h; | 96% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
913841-86-8
N-[3-chloromethyl-3H-thiazol-2-(E/Z)ylidene]-4-(3,3-dimethylbutyrylamino)-3,5-difluorobenzamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3,3-dimethylbutyrylamino)-3,5-difluoro-N-thiazol-2-ylbenzamide With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; Stage #2: carbonochloridic acid, chloromethyl ester In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; | 96% |
| Stage #1: 4-(3,3-dimethylbutyrylamino)-3,5-difluoro-N-thiazol-2-ylbenzamide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1.5h; Stage #2: carbonochloridic acid, chloromethyl ester In N,N-dimethyl-formamide at 20℃; | 86% |
-
-
87184-99-4
4-(tert-butyldimethylsiloxy)-1-butanol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 2h; | 96% |
-
-
100-02-7
4-nitro-phenol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
50780-50-2
4-chloromethoxycarbonyloxy-1-nitrobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 1h; | 95% |
| With 4-methyl-morpholine In dichloromethane at 0 - 25℃; | 94% |
| With TEA Ambient temperature; | 90% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
502158-15-8
(S)-2-[4-(Chloromethoxycarbonylamino-methyl)-benzoylamino]-propionic acid benzyl ester

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane at 0℃; | 95% |
-
-
2462-34-2
L-valine benzyl ester hydrochloride

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 0.5h; | 95% |
-
-
177339-15-0
3-[(1-dimethylamino-2-methyl)prop-2-yl]phenol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
177339-27-4
3-[(1-dimethylamino-2-methyl)prop-2-yl]phenyl chloromethyl carbonate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; triethylamine In dichloromethane | 95% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
160738-57-8, 112811-59-3
gatifloxacin

-
-
925684-58-8
7-(4-(2-chloromethoxycarbonyl)-3-methylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxoquinlone-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In dichloromethane for 4h; Inert atmosphere; | 95% |
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In dichloromethane at 0℃; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 0.5h; Inert atmosphere; Cooling with ice; | 95% |
| Stage #1: nimodipin With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: carbonochloridic acid, chloromethyl ester In tetrahydrofuran at 20℃; | 94.84% |
-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 95% |
Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
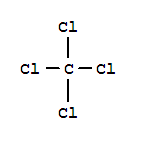


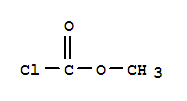






 T
T