-
Name
Chloromethyl chloroformate
- EINECS 244-793-3
- CAS No. 22128-62-7
- Article Data17
- CAS DataBase
- Density 1.468 g/cm3
- Solubility Slightly miscible with water.
- Melting Point <-20 °C
- Formula C2H2Cl2O2
- Boiling Point 107.9 °C at 760 mmHg
- Molecular Weight 128.943
- Flash Point 25.5 °C
- Transport Information UN 2745 6.1/PG 2
- Appearance Colorless liquid
- Safety 26-36/37/39-45
- Risk Codes 23-34
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Formicacid, chloro-, chloromethyl ester (6CI,8CI);Chloroformic acid chloromethylester;Chloromethoxycarbonyl chloride;Chloromethyl carbonochloridate;UN2745;
- PSA 26.30000
- LogP 1.55810
Synthetic route
-
-
79-22-1
methyl chloroformate

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With chlorine at 65 - 88℃; for 4h; Yields of byproduct given; | A n/a B 22% |
| With chlorine at 65 - 88℃; for 4h; Yield given. Yields of byproduct given; | |
| With 2,2'-azobis(isobutyronitrile); chlorine at 70℃; for 9.5h; | A 1.94 %Chromat. B 68.5 %Chromat. |
-
-
107-31-3
Methyl formate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| bei gemaessigter Chlorierung am Licht; |
-
-
107-31-3
Methyl formate

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
C
-
503-38-8
trichloromethyl chloroformate

| Conditions | Yield |
|---|---|
| Bei der Chlorierung im Licht; | |
| With oxygen; chlorine at 22.85℃; Kinetics; Further Variations:; Reagents; |

-
-
56-23-5
tetrachloromethane

-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| at 25℃; Kinetics; Photochlorierung; |
-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| bei gemaessigter Chlorierung am Licht; | |
| With chlorine Ambient temperature; Irradiation; | |
| With sulfuryl dichloride; Perbenzoic acid Heating; |

| Conditions | Yield |
|---|---|
| benzyltri(n-butyl)ammonium chloride at 0 - 20℃; for 0.5h; |
-
-
7782-50-5
chlorine

-
-
79-22-1
methyl chloroformate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| at 23.5 - 27℃; durch UV-Licht (λ:435.8 nm) initiierten Reaktion in der Dampfphase; |
-
-
107-31-3
Methyl formate

-
-
7782-50-5
chlorine

-
A
-
22128-63-8
dichloromethoxycarbonyl chloride

-
B
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| im diffusen Licht; |

-
-
123-75-1
pyrrolidine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
93765-67-4
N-(chloromethyloxycarbonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 2h; Substitution; | 100% |
| With pyridine Yield given; |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
100-61-8
N-methylaniline

-
-
186353-05-9
[N-methyl-N-phenyl]carbamic acid chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 100% |
| With triethylamine In dichloromethane at 0 - 5℃; for 4h; | 73% |
-
-
44897-15-6
sarcosine diethylamide

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
934548-22-8
N-(chloromethyloxycarbonyl)sarcosine diethylamide

| Conditions | Yield |
|---|---|
| In dichloromethane at -10 - 20℃; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; for 0.75h; | 100% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane; water at 20℃; for 2h; | 100% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
108-98-5
thiophenol

-
-
133217-39-7
Thiocarbonic acid O-chloromethyl ester S-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether 1) 0 to 5 deg C, 30 min, 2) RT, 16h; | 99% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
158991-23-2
PD 154075

-
-
247017-85-2
3-[(R)-2-(Benzofuran-2-ylmethoxycarbonylamino)-2-((S)-1-phenyl-ethylcarbamoyl)-propyl]-indole-1-carboxylic acid chloromethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: PD 154075 With potassium hexamethylsilazane In tetrahydrofuran at -78℃; Metallation; Stage #2: carbonochloridic acid, chloromethyl ester In tetrahydrofuran at -78℃; Acylation; | 99% |
-
-
112-35-6
triethylene glucol monomethyl ether

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
209551-63-3
chloromethyl (2-(2-(2-methoxyethoxy)ethoxy)ethyl) carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 22h; Inert atmosphere; | 99% |
| With pyridine In dichloromethane at 0 - 20℃; | 99% |
| With pyridine In dichloromethane | |
| With pyridine In dichloromethane at 20℃; | |
| With pyridine In dichloromethane at -78℃; for 3h; |
-
-
110-91-8
morpholine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
93765-68-5
chloromethyl morpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 0.5h; | 99% |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.58333h; |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 99% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In 1,2-dichloro-ethane; N,N-dimethyl-formamide at 60℃; for 1.08333h; | 99% |
-
-
107-03-9
1-thiopropane

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tert-butyl methyl ether at 5 - 20℃; | 99% |
| With N-ethyl-N,N-diisopropylamine In tert-butyl methyl ether at 5 - 20℃; | 29 g |
-
-
32399-12-5
2-(N-methylamino)-3-hydroxymethylpyridine

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 5℃; for 0.0833333h; | 99% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -10℃; for 1h; |
-
-
1557084-43-1
3-(2-chloro-4-morpholinopyrido[3,2-d]pyrimidin-7-yl)aniline

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
1557084-82-8
chloromethyl (3-(2-chloro-4-morpholinopyrido[3,2-d]pyrimidin-7-yl)phenyl)carbamate

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In chloroform at 20℃; Cooling with ice; | 98.4% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
67-63-0
isopropyl alcohol

-
-
35180-01-9
chloromethyl isopropyl carbonate

| Conditions | Yield |
|---|---|
| With triethylamine at 0℃; | 98% |
| With pyridine In diethyl ether; isopropyl alcohol at 10℃; for 18h; Cooling; | 95% |
| With pyridine In diethyl ether at 0 - 20℃; | 92% |
-
-
111-87-5
octanol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
920967-14-2
chloromethyl-1-octyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 98% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane | |
| With triethylamine In dichloromethane at 0 - 20℃; for 21h; |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at -40 - 20℃; for 0.333333h; | 98% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
109-89-7
diethylamine

-
-
133217-92-2
chloromethyl N,N-diethyl carbamate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 0.5h; | 97% |
| In hexane at -10 - -5℃; for 2.25h; | 92% |
| With triethylamine In diethyl ether 1) 0 to 5 deg C, 30 min, 2) RT, 16h; | 71% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
111-27-3
hexan-1-ol

-
-
663597-51-1
chloromethyl hexyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 25℃; | 97% |
| With pyridine In dichloromethane at 0 - 20℃; for 20h; | 63% |
-
-
90-15-3
α-naphthol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
132905-86-3
Carbonic acid chloromethyl ester naphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 5℃; for 1.66667h; | 96% |

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
913841-86-8
N-[3-chloromethyl-3H-thiazol-2-(E/Z)ylidene]-4-(3,3-dimethylbutyrylamino)-3,5-difluorobenzamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3,3-dimethylbutyrylamino)-3,5-difluoro-N-thiazol-2-ylbenzamide With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; Stage #2: carbonochloridic acid, chloromethyl ester In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; | 96% |
| Stage #1: 4-(3,3-dimethylbutyrylamino)-3,5-difluoro-N-thiazol-2-ylbenzamide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1.5h; Stage #2: carbonochloridic acid, chloromethyl ester In N,N-dimethyl-formamide at 20℃; | 86% |
-
-
87184-99-4
4-(tert-butyldimethylsiloxy)-1-butanol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 2h; | 96% |
-
-
100-02-7
4-nitro-phenol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
50780-50-2
4-chloromethoxycarbonyloxy-1-nitrobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 1h; | 95% |
| With 4-methyl-morpholine In dichloromethane at 0 - 25℃; | 94% |
| With TEA Ambient temperature; | 90% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
502158-15-8
(S)-2-[4-(Chloromethoxycarbonylamino-methyl)-benzoylamino]-propionic acid benzyl ester

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane at 0℃; | 95% |
-
-
2462-34-2
L-valine benzyl ester hydrochloride

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 0.5h; | 95% |
-
-
177339-15-0
3-[(1-dimethylamino-2-methyl)prop-2-yl]phenol

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
177339-27-4
3-[(1-dimethylamino-2-methyl)prop-2-yl]phenyl chloromethyl carbonate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; triethylamine In dichloromethane | 95% |
-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

-
-
160738-57-8, 112811-59-3
gatifloxacin

-
-
925684-58-8
7-(4-(2-chloromethoxycarbonyl)-3-methylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxoquinlone-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In dichloromethane for 4h; Inert atmosphere; | 95% |
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In dichloromethane at 0℃; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 0.5h; Inert atmosphere; Cooling with ice; | 95% |
| Stage #1: nimodipin With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: carbonochloridic acid, chloromethyl ester In tetrahydrofuran at 20℃; | 94.84% |
-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

-
-
22128-62-7
carbonochloridic acid, chloromethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 95% |
Chloromethyl chloroformate Specification
The Chloromethyl chloroformate with CAS registry number of 22128-62-7 is also known as Carbonochloridic acid, chloromethyl ester. The IUPAC name is Chloromethyl carbonochloridate. It belongs to product categories of Acid HalidesDerivatization Reagents HPLC; Carbonyl ChloridesDerivatization Reagents HPLC; Carbonyl Compounds; Fluorescence; Halogenated; Organic Building Blocks. Its EINECS registry number is 244-793-3. In addition, the formula is C2H2Cl2O2 and the molecular weight is 128.94. This chemical is a colorless liquid that at low levels cause damage to health. What's more, it can be used as medicine and pesticide intermediate, and it should be stored at the temperature of 2-8 °C away from oxidants, water and alkali.
Physical properties about Chloromethyl chloroformate are: (1)ACD/LogP: 1.14; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.14; (4)ACD/LogD (pH 7.4): 1.14; (5)ACD/BCF (pH 5.5): 4.34; (6)ACD/BCF (pH 7.4): 4.34; (7)ACD/KOC (pH 5.5): 99.51; (8)ACD/KOC (pH 7.4): 99.51; (9)#H bond acceptors: 2; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.432; (12)Molar Refractivity: 22.78 cm3; (13)Molar Volume: 87.8 cm3; (14)Surface Tension: 34.8 dyne/cm; (15)Density: 1.468 g/cm3; (16)Flash Point: 25.5 °C; (17)Enthalpy of Vaporization: 34.68 kJ/mol; (18)Boiling Point: 107.9 °C at 760 mmHg; (19)Vapour Pressure: 26.5 mmHg at 25 °C.
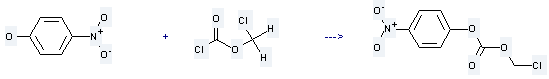
Uses of Chloromethyl chloroformate: it is used to produce carbonic acid chloromethyl ester 4-nitro-phenyl ester by reaction with 4-nitro-phenol. The reaction occurs with reagent Et3N and solvent CH2Cl2 at the temperature of 5 °C for 100 minutes. The yield is about 88.2 %.
When you are using this chemical, please be cautious about it. As a chemical, it is toxic by inhalation and causes burns. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If accident happens or you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C(OC(=O)Cl)Cl
2. InChI: InChI=1S/C2H2Cl2O2/c3-1-6-2(4)5/h1H2
3. InChIKey: JYWJULGYGOLCGW-UHFFFAOYSA-N
Related Products
- Chloromethyl 2-methylpropanoate
- Chloromethyl 4-chlorophenyl sulfide
- Chloromethyl acetate
- CHLOROMETHYL BISMUTHINE
- Chloromethyl butyrate
- Chloromethyl chloroformate
- Chloromethyl chlorosulfate
- Chloromethyl ethyl carbonate
- Chloromethyl ethyl ether
- Chloromethyl isopropyl carbonate
- 221290-14-8
- 221295-04-1
- 221297-82-1
- 221313-10-6
- 22131-92-6
- 221322-07-2
- 221322-09-4
- 22133-95-5
- 22134-07-2
- 22134-11-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View