Refine
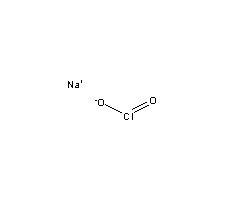
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route
Conditions

| Conditions | Yield |
|---|---|
| With water at 500-600°C; with oxygen containing 0.001g H2O/l; | 100% |
| With water at 400°C with oxygen containing 0.001g H2O/l; | 94.6% |
| With water at 750°C with oxygen containing 0.001g H2O/l; | 61.9% |
Conditions

| Conditions | Yield |
|---|---|
| With oxygen -35°C; | A 7.5% B 92.5% |
| With oxygen -50°C; | A 9.1% B 90.9% |
| With oxygen at low temp.; |
Conditions

| Conditions | Yield |
|---|---|
| In ammonia conducting oxygen into Ba soln. in NH3 liq.; | A 9.07% B n/a |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| Kinetics; byproducts: oxygen; Ba-atoms and O3-molecules are brought to reaction in crossed molecular beams to form BaO2 and O-atoms at high collision energies. Lower collision energies lead to BaO and O2 as products.; Reaction products are detected by quadrupole mass spectrometry.; |
Conditions

| Conditions | Yield |
|---|---|
| In not given Electrolysis; electrolysis of Ba(OH)2-solution at 0-12°C at using of a diaphragma; the used solution is treated before and during the electrolysis with O3-containing O2 or ozonated CO2-free air; BaO2 ppts.;; | |
| In not given Electrolysis; electrolysis of Ba(OH)2-solution at 0-12°C at using of a diaphragma; the used solution is treated before and during the electrolysis with O3-containing O2 or ozonated CO2-free air; BaO2 ppts.;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With pyrographite exclusion of air, annealing while passing steam into react. mixt.; | |
| With pyrographite exclusion of air, annealing while passing steam into react. mixture; | |
| With pyrographite exclusion of air, annealing while passing steam into react. mixt.; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| gentle glowing; | |
| heating; |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) inert conditions, thermal decompg. of BaCO3 (1373 K, 10**(-2) Pa, 100 h), then dry O2 atmosphere (623 -673 K, 100 h, several regrindings); detd. by X-ray diffraction; | |
| With air In neat (no solvent) heating of a mixture of BaCO3 and soot forms BaO; treatment of the hot BaO with air;; |
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In neat (no solvent) Cl2 reacts with BaO at grinding under formation of an instable addition-compound which decomposites to BaO2, BaCl2 and a trace of Ba(ClO)2;; | A <1 B n/a C n/a |
| With Cl2 In neat (no solvent) Cl2 reacts with BaO at grinding under formation of an instable addition-compound which decomposites to BaO2, BaCl2 and a trace of Ba(ClO)2;; | A <1 B n/a C n/a |
...Expand


-
A
-
1304-29-6
barium peroxide

-
B
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper
Conditions

| Conditions | Yield |
|---|---|
| With copper(II) oxide glowing; | |
| With copper(II) oxide glowing; dry reactants; | A 0% B n/a |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| With detonating gas 250-280°C; | |
| With oxygen In neat (no solvent) reaction of BaO with O2 under formation of BaO2;; | |
| With air; water at 500-600°C and normal pressure; |
Conditions

| Conditions | Yield |
|---|---|
| With air; water 500°C; | |
| With air; H2O 500°C; |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) reation of N2O with BaO at 480-620°C forms BaO2 and Ba(NO2)2;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating a mixture of 3 mol BaO and 1 mol Ag2O in a stream of N2 at 300-400°C for several days;; | |
| In neat (no solvent) heating a mixture of 3 mol BaO and 1 mol Ag2O in a stream of N2 at 300-400°C for several days;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With hydrogen In not given reaction at 250°C;; | |
| With H2 In not given reaction at 250°C;; |
Conditions

| Conditions | Yield |
|---|---|
| byproducts: KCl; gentle glowing; leaching off KCl from the cooled melt (water); hydrated BaO2; |
Conditions

| Conditions | Yield |
|---|---|
| In melt equal wt. amts.; | A 0% B n/a |
| In melt equal wt. amts.; | A 0% B n/a |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With Al2O3 In neat (no solvent) heating (air, 300 K/h to 1073 K, 5 h at 1073 K), slowly cooling to 923 K (4.2 K/h); |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air burning; |
Conditions

| Conditions | Yield |
|---|---|
| burning; | |
| With water 500°C; with dry oxygen; | <1 |
-
-
12337-90-5
barium peroxide * hydrogen peroxide

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| careful drying; | |
| careful drying; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| 100°C; 2 wk standing above P2O5 in vac. at 350°C; | |
| above 200°C; | |
| careful drying; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| heating; | |
| heating; | |
| With O2 In water precipitation from oxygenated water; XRD; |
Conditions

| Conditions | Yield |
|---|---|
| With NH3 In water pptn. on addn. of NH3 and H2O2 to aq. Ba(NO3)2; drying (O2 flow, 200°C); |
Conditions

| Conditions | Yield |
|---|---|
| In water boiling of a solution of Ba(OH)2*8H2O in H2O for a longer period of time; addition of 3% H2O2-solution ppts. BaO2*8H2O; sucking off and washing; draining over P2O5 at roomtemperature;; contains 98.87-99.33% BaO2;; | |
| In water filtration of a solution of Ba(OH)2*8H2O in destillated H2O in CO2-free O2-atmosphere; addition of 1% H2O2-solution ppts. BaO2*8H2O; sucking off, washing and drying at 95°C for 5 min in CO2-free O2; draining in vac. at 90°C for 20 min;; content: 97.4% BaO2, 1.4% BaO and 1.2% H2O + CO2;; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| byproducts: H2O; at 700°C in pure dry oxygen stream; pure prod. with little hydroxide content; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| careful drying; | |
| careful drying; |
Conditions

| Conditions | Yield |
|---|---|
| With barium amalgam not very dry oxygen; | A <1 B n/a C n/a D n/a |
...Expand

-
-
80937-33-3
oxygen

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| With barium amalgam 10h, 60 atm, 15°C; | |
| With barium amalgam >10h, 60 atm, 15°C; or 1h, 60 atm, 100°C; | 0% |
Conditions

| Conditions | Yield |
|---|---|
| In melt blowing air or oxygen into the melt at 400-600°C; sieving in melt; | |
| 10h, 60 atm, 15°C; | 0% |
| In water Electrolysis; blowing oxygen into a cooled soln.; | |
| In water Electrolysis; blowing oxygen into a cooled soln.; |
-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| 1h at 1150°C in electric oven; | 100% |
| With metals heating; | |
| iron(III) oxide byproducts: oxygen; beginning of oxygen formation at 200-250°C; |

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; the mixt. of the oxides was placed in a pyrophyllite container with a graphite heater lined with a Pt foil in a toroidal high-pressure chamber; 1.0 GPa, 940°C, 10 min.;; | 100% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) (Ar or vac.); heated to 900°C at a rate of 200°C/h, kept at 900°C for 20 h, cooled to 500°C at a rate of 5°C/h; allowed to cool to room temp.; | A 1% B 1% C 99% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In solid heating mixt. of educts in air, O2 or N2 atm. at 450 °C, 10 min.;; | 95% |
| In neat (no solvent, solid phase) heating mixt. of educts in air, O2 or N2 atm. at 450 °C, 10 min.;; | 95% |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) a mixt. of 1BaCO3 + 1BaO2 + 0.5Y2O3 + 1 Pd is heated in a silica crucible at 815°C (22 h); at temps. above 850°C, YBa2Cu(3-x)Pd(x)O(y) is decomposed; at temps. below 800°C, BaCuO2 is the major phase; Cu(3+) content detd. by chem. anal.; | A n/a B n/a C 90% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) heated in alumina crucibles at 815°C; | 90% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air explosive compression of a powder mixture of the reaction components; | A 80% B 10% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| 96% of the theoretically necessary Al; 80% BaO2 refer to used Al amt.; Al content: <0.1%; | 74.3% |
| 96% of the theoretically necessary Al; 80% BaO2 refer to used Al amt.; Al content: <0.1%; | 74.3% |
| 96% of the theoretically necessary Al; 30% BaO2 refer to used Al amt.; Al content: <.1%; | 65% |
| 96% of the theoretically necessary Al; 30% BaO2 refer to used Al amt.; Al content: <.1%; | 65% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air explosive compression of a powder mixture of the reaction components; | A 70% B 20% |
...Expand


-
-
1304-29-6
barium peroxide

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper
Conditions

| Conditions | Yield |
|---|---|
| With oxygen In solid solid combustion synthesis, mixed, pressed, ignited under O2 atmosphere, powdered and recompacted products resintered; | A 35% B 65% |
| With oxygen In solid solid combustion synthesis, mixed, pressed, ignited under O2 atmosphere; BaCuO2: main product; | A n/a B 30% |
| With oxygen In solid pellets fired twice in the conventional way; | A 5% B n/a |
...Expand


-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; the mixt. of the oxides was placed in a pyrophyllite container with a graphite heater lined with a Pt foil in a toroidal high-pressure chamber; 1.0 GPa, 940°C, 10 min.;; | A n/a B n/a C 60% |
| With Cu In neat (no solvent) High Pressure; the mixt. of the oxides was placed in a pyrophyllite container with a graphite heater lined with a Cu foil in a toroidal high-pressure chamber; 2.0 GPa, 940°C, 10 min.;; | A n/a B n/a C 10% |
| With Cu In neat (no solvent) High Pressure; the mixt. of the oxides was placed in a pyrophyllite container with a graphite heater lined with a Cu foil in a toroidal high-pressure chamber; 1.0 GPa, 940°C, 10 min.;; | A n/a B n/a C 5% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air explosive compression of a powder mixture of the reaction components; | A 40% B 50% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| after 3 h; | 40% |
| after 3 h; | 40% |
...Expand

-
-
1304-29-6
barium peroxide
Conditions

| Conditions | Yield |
|---|---|
| With air explosive compression; | 30% |
| With alkali carbonate In water byproducts: alkali peroxide; | |
| With alkali carbonate In water byproducts: alkali peroxide; |
Conditions

| Conditions | Yield |
|---|---|
| mechanical activation, annealing at 1200°C; detd. by X-ray diffraction and scanning electron microscopy; | A 1% B n/a C n/a |
| mechanical activation, annealing at 1100 or 1300°C; detd. by X-ray diffraction and scanning electron microscopy; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air In neat (no solvent) heating of components at 850-900°C for 48 h; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, samples are fired in air in a temp. range of 900 to 935°C; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, attempts are made under a moderate flow of N2 with one end of the furnace tube open to the air, firing the samples in a temp. range of 900 to 935°C several times to ensure homogenity and phase purity; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, attempts are made under a moderate flow of N2 with one end of the furnace tube open to the air, firing the samples in a temp. range of 900 to 935°C several times to ensure homogenity and phase purity; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, firing the samples in air in a temp. range of 900 to 935°C several times to ensure homogenity and phase purity; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, sample is fired in air in a temp. range of 900 to 935°C; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With air used an alumina crucible with a lid, attempts are made under a moderate flow of N2 with one end of the furnace tube open to the air, firing the samples in a temp. range of 900 to 935°C several times to ensure homogenity and phase purity; powder x-ray diffraction; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) further educt CuO; appropriate amts. BaO2, SrCO3, Y2O3, CaO and CuO mixed, sintered twice (930°C, 12, air), cooled, PbO mixed in, heated twice (830°C, 12h, 1%O2-N2); quenched (N2), sintered (740-830°C, 40h, 1%O2-N2), quenched (N2); impurity phase = BaPbO3 (X-ray diffraction), EDX, iodide titration for amt. O; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) further educt CuO; appropriate amts. BaO2, SrCO3, Y2O3, CaO and CuO mixed, sintered twice (930°C, 12, air), cooled, PbO mixed in, heated twice (830°C, 12h, 1%O2-N2); quenched (N2), sintered (740-830°C, 40h, 1%O2-N2), quenched (N2); single phase (X-ray diffraction), EDX, iodide titration for amt. O; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) further educt CuO; appropriate amts. BaO2, SrCO3, Y2O3, CaO and CuO mixed, sintered twice (930°C, 12, air), cooled, PbO mixed in, heated twice (830°C, 12h, 1%O2-N2); quenched (N2), sintered (740-830°C, 40h, 1%O2-N2), quenched (N2); single phase (X-ray diffraction), EDX, iodide titration for amt. O; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) further educt CuO; appropriate amts. BaO2, SrCO3, Y2O3, CaO and CuO mixed, sintered twice (930°C, 12, air), cooled, PbO mixed in, heated twice (830°C, 12h, 1%O2-N2); quenched (N2), sintered (740-830°c, 40h, 1%O2-N2), quenched (N2)L; single phase (X-ray diffraction), EDX, iodide titration for amt. O; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) further educt CuO; appropriate amts. BaO2, SrCO3, Y2O3, CaO and CuO mixed, sintered twice (930°C, 12, air), cooled, PbO mixed in, heated twice (830°C, 12h, 1%O2-N2); quenched (N2), sintered (785 or 830°lC, 40h, 1%O2-N2), quenched (N2); EDX, iodide titration for amt. O; |
...Expand

Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View







 O,
O, Xn
Xn