-
Name
1,1-Diethoxybutane
- EINECS 222-913-5
- CAS No. 3658-95-5
- Article Data32
- CAS DataBase
- Density 0.841 g/cm3
- Solubility
- Melting Point
- Formula C8H18O2
- Boiling Point 142.999 °C at 760 mmHg
- Molecular Weight 146.23
- Flash Point 28.914 °C
- Transport Information
- Appearance Clear colorless liquid
- Safety 26-37/39-16
- Risk Codes 10-36/38
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms Butyraldehyde,diethyl acetal (6CI,7CI,8CI);Butanal diethyl acetal;Butylaldehyde diethyl acetal;Butyraldehyde ethyl acetal;
- PSA 18.46000
- LogP 2.18560
1,1-Diethoxybutane Specification
The 1,1-Diethoxybutane, with the CAS registry number 3658-95-5, is also known as Butyraldehyde diethyl acetal. Its EINECS registry number is 222-913-5. This chemical's molecular formula is C8H18O2 and molecular weight is 146.23. What's more, its IUPAC name is the same with its product name.
Physical properties about 1,1-Diethoxybutane are: (1)ACD/LogP: 2.041; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.04; (4)ACD/LogD (pH 7.4): 2.04; (5)ACD/BCF (pH 5.5): 20.94; (6)ACD/BCF (pH 7.4): 20.94; (7)ACD/KOC (pH 5.5): 307.01; (8)ACD/KOC (pH 7.4): 307.01; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 18.46 Å2; (13)Index of Refraction: 1.405; (14)Molar Refractivity: 42.561 cm3; (15)Molar Volume: 173.691 cm3; (16)Polarizability: 16.872×10-24cm3; (17)Surface Tension: 24.684 dyne/cm; (18)Density: 0.842 g/cm3; (19)Flash Point: 28.914 °C; (20)Enthalpy of Vaporization: 36.445 kJ/mol; (21)Boiling Point: 142.999 °C at 760 mmHg; (22)Vapour Pressure: 6.833 mmHg at 25 °C.
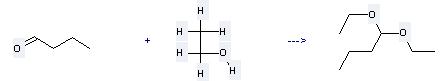
Preparation of 1,1-Diethoxybutane: this chemical can be prepared by butyraldehyde with ethanol. This reaction needs reagent CaCl2 at ambient temperature. The yield is 72 %.
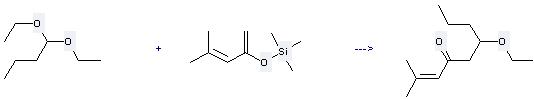
Uses of 1,1-Diethoxybutane: it is used to produce other chemicals. For example, it can react with 4-Methyl-2-trimethylsiloxy-1,3-pentadien to get 6-ethoxy-2-methyl-non-2-en-4-one. The reaction occurs with catalyzer stannic chloride. The yield is 61 %.
You can still convert the following datas into molecular structure:
(1) SMILES: O(CC)C(OCC)CCC
(2) InChI: InChI=1S/C8H18O2/c1-4-7-8(9-5-2)10-6-3/h8H,4-7H2,1-3H3
(3) InChIKey: UVHXZFGCCJLFMX-UHFFFAOYSA-N
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 36589-61-4
- 36590-19-9
- 36590-52-0
- 36592-77-5
- 36596-67-5
- 36598-30-8
- 36598-31-9
- 365996-05-0
- 365996-06-1
- 365996-07-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View