-
Name
1-Butyl-3-methylimidazolium hexafluorophosphate
- EINECS 678-095-9
- CAS No. 174501-64-5
- Article Data103
- CAS DataBase
- Density 1.38 g/mL at 20 °C(lit.)
- Solubility non-water soluble
- Melting Point 6.5 °C
- Formula C8H15N2.PF6
- Boiling Point >340°C
- Molecular Weight 284.185
- Flash Point >350°C
- Transport Information
- Appearance Clear pale yellow oil
- Safety 26-37/39
- Risk Codes 36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 1H-Imidazolium, 1-butyl-3-methyl-, hexafluorophosphate(1-) (9CI);3-Butyl-1-methylimidazolium hexafluorophosphate;1-Butyl-3-methyl-1H-imidazolium hexafluorophosphate;1-Butyl-3-methyl-1H-imidazolium hexafluorophosphate(1-);1-Methyl-3-butyl imidazolium hexafluorophosphate;1-n-Butyl-3-methylimidazolium hexafluorophosphate;BMI-PF 6;BmimPF6;BMIm hexafluorophosphate(1-);Bmim hexafluorophosphate;N-Butyl-N'-methylimidazolium hexafluorophosphate;LP 104;
- PSA 22.40000
- LogP 4.49510
Synthetic route
-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With Amberlyst A-26 (PF6- form) In methanol | 100% |
| With sodium hexaflorophosphate In acetone at 80℃; for 0.166667h; microwave irrradiation; | 99% |
| With potassium hexafluorophosphate In acetone at 20℃; for 24h; | 98% |
-
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate In water at 20℃; | 100% |
| With ammonium hexafluorophosphate In water at 20℃; for 2h; | 91% |
| With potassium hexafluorophosphate In water at 20℃; for 24h; | 87% |
-
-
65039-05-6
1-methyl-3-(n-butyl)imidazolium iodide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With Amberlyst A-26 (PF6- form) In methanol | 100% |
| With Amberlist A-26 BF6(-) form In methanol Ionic liquid; |
-
-
4316-42-1
1-Butylimidazole

-
-
149-73-5
trimethyl orthoformate

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate Reagent/catalyst; Schlenk technique; Reflux; | 95% |
| With ammonium hexafluorophosphate at 110℃; for 17h; Reagent/catalyst; Inert atmosphere; | 88% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
109-65-9
1-bromo-butane

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate at 40 - 120℃; for 0.166667h; microwave irradiation; sonication; | 94% |
| With potassium hexafluorophosphate at 80℃; for 3.5h; | 93% |
| With ammonium hexafluorophosphate; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 80℃; for 15h; | 89% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
109-69-3
n-Butyl chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With sodium hexaflorophosphate at 70℃; for 336h; | 92% |
| Stage #1: 1-methyl-1H-imidazole; n-Butyl chloride In toluene for 48h; Reflux; Stage #2: With potassium hexafluorophosphate In toluene | 92% |
| With potassium hexafluorophosphate at 120 - 180℃; for 0.75h; microwave irradiation; | 90% |
-
-
4316-42-1
1-Butylimidazole

-
-
77-78-1
dimethyl sulfate

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 1-Butylimidazole; dimethyl sulfate for 0.25h; Stage #2: With sodium hexaflorophosphate In water | 92% |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate In acetone for 24h; | 90% |
-
-
67-56-1
methanol

-
-
671779-18-3
N-methyl-N'-n-butylimidazolium-2-carboxylate

-
A
-
14660-45-8
potassium monomethylcarbonate

-
B
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate at 20℃; for 12h; | A 87% B 72% |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
542-69-8
1-iodo-butane

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 80℃; for 10h; | 85% |

-
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 75% |

-
-
121-43-7
Trimethyl borate

-
-
7664-39-3
hydrogen fluoride

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
D
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: potassium fluoride; N-trimethylsilyl-tris(pentafluorethyl)phosphazen; Trimethyl borate In 1,2-dimethoxyethane at 20 - 60℃; for 1h; Stage #2: hydrogen fluoride In 1,2-dimethoxyethane; water at 0 - 20℃; for 3h; Stage #3: 1-butyl-3-methylimidazolium chloride In water | A 60% B 30% C 6% D 4% |

-
-
4316-42-1
1-Butylimidazole

-
-
616-38-6
carbonic acid dimethyl ester

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 1-Butylimidazole; carbonic acid dimethyl ester In methanol at 135℃; for 7h; Stage #2: With sodium hexaflorophosphate In methanol; acetone at 25℃; for 2h; | 52% |
-
-
4316-42-1
1-Butylimidazole

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 - 70 °C 2: NH4PF6 View Scheme |

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| at 20℃; |
-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
109-73-9
N-butylamine

-
-
74-89-5
methylamine

-
A
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; N-butylamine; methylamine In water at 0℃; Stage #2: With hexafluorophosphoric acid In water at 0℃; Stage #3: Glyoxal In water at 0 - 20℃; |


-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With sodium hexaflorophosphate |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With alkali metal hexafluorophosphate at 150℃; for 0.5h; Microwave irradiation; Cooling; neat (no solvent); |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 24h; |

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In water | |
| Ionic liquid; Inert atmosphere; |
-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With Amberlyst A-26 (PF6- form) In methanol | 100% |
| With sodium hexaflorophosphate In acetone at 80℃; for 0.166667h; microwave irrradiation; | 99% |
| With potassium hexafluorophosphate In acetone at 20℃; for 24h; | 98% |
-
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate In water at 20℃; | 100% |
| With ammonium hexafluorophosphate In water at 20℃; for 2h; | 91% |
| With potassium hexafluorophosphate In water at 20℃; for 24h; | 87% |
-
-
65039-05-6
1-methyl-3-(n-butyl)imidazolium iodide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With Amberlyst A-26 (PF6- form) In methanol | 100% |
| With Amberlist A-26 BF6(-) form In methanol Ionic liquid; |
-
-
4316-42-1
1-Butylimidazole

-
-
149-73-5
trimethyl orthoformate

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate Reagent/catalyst; Schlenk technique; Reflux; | 95% |
| With ammonium hexafluorophosphate at 110℃; for 17h; Reagent/catalyst; Inert atmosphere; | 88% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
109-65-9
1-bromo-butane

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate at 40 - 120℃; for 0.166667h; microwave irradiation; sonication; | 94% |
| With potassium hexafluorophosphate at 80℃; for 3.5h; | 93% |
| With ammonium hexafluorophosphate; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 80℃; for 15h; | 89% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
109-69-3
n-Butyl chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With sodium hexaflorophosphate at 70℃; for 336h; | 92% |
| Stage #1: 1-methyl-1H-imidazole; n-Butyl chloride In toluene for 48h; Reflux; Stage #2: With potassium hexafluorophosphate In toluene | 92% |
| With potassium hexafluorophosphate at 120 - 180℃; for 0.75h; microwave irradiation; | 90% |
-
-
4316-42-1
1-Butylimidazole

-
-
77-78-1
dimethyl sulfate

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 1-Butylimidazole; dimethyl sulfate for 0.25h; Stage #2: With sodium hexaflorophosphate In water | 92% |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate In acetone for 24h; | 90% |
-
-
67-56-1
methanol

-
-
671779-18-3
N-methyl-N'-n-butylimidazolium-2-carboxylate

-
A
-
14660-45-8
potassium monomethylcarbonate

-
B
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate at 20℃; for 12h; | A 87% B 72% |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
542-69-8
1-iodo-butane

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 80℃; for 10h; | 85% |

-
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 75% |

-
-
121-43-7
Trimethyl borate

-
-
7664-39-3
hydrogen fluoride

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
D
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: potassium fluoride; N-trimethylsilyl-tris(pentafluorethyl)phosphazen; Trimethyl borate In 1,2-dimethoxyethane at 20 - 60℃; for 1h; Stage #2: hydrogen fluoride In 1,2-dimethoxyethane; water at 0 - 20℃; for 3h; Stage #3: 1-butyl-3-methylimidazolium chloride In water | A 60% B 30% C 6% D 4% |

-
-
4316-42-1
1-Butylimidazole

-
-
616-38-6
carbonic acid dimethyl ester

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 1-Butylimidazole; carbonic acid dimethyl ester In methanol at 135℃; for 7h; Stage #2: With sodium hexaflorophosphate In methanol; acetone at 25℃; for 2h; | 52% |
-
-
4316-42-1
1-Butylimidazole

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 - 70 °C 2: NH4PF6 View Scheme |

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| at 20℃; |
-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
109-73-9
N-butylamine

-
-
74-89-5
methylamine

-
A
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; N-butylamine; methylamine In water at 0℃; Stage #2: With hexafluorophosphoric acid In water at 0℃; Stage #3: Glyoxal In water at 0 - 20℃; |


-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With sodium hexaflorophosphate |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With alkali metal hexafluorophosphate at 150℃; for 0.5h; Microwave irradiation; Cooling; neat (no solvent); |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 24h; |

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In water | |
| Ionic liquid; Inert atmosphere; |
-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

| Conditions | Yield |
|---|---|
| With Amberlyst A-26 (Cl- form) In methanol | 100% |
-
-
30169-25-6
3,6-bis-(3,5-dimethyl-pyrazol-1-yl)-1,2,4,5-tetrazine

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate With potassium carbonate In acetonitrile for 0.666667h; Heating; Stage #2: 3,6-bis-(3,5-dimethyl-pyrazol-1-yl)-1,2,4,5-tetrazine In acetonitrile Heating; Further stages.; | 99% |
-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
-
119393-94-1
1-butyl-3-methyl-1H-imidazole-2(3H)-thione

| Conditions | Yield |
|---|---|
| Stage #1: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 20℃; Electrolysis; Inert atmosphere; Stage #2: With sulfur for 0.166667h; Inert atmosphere; Irradiation; | 99% |
-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
-
586965-19-7
1-butyl-3-methyl-2-imidazolone

| Conditions | Yield |
|---|---|
| With superoxide radical anion at 25℃; under 742.574 Torr; Kinetics; Inert atmosphere; | 95% |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In toluene byproducts: H2O; Au complex and (C3H3N2(CH3)C4H9)PF6 added to toluene, stirred at 25°C for 14 h; pptd. (pentane), collected on a frit, washed (pentane), dried (vac.); elem. anal.; | 95% |
-
-
1079-66-9
chloro-diphenylphosphine

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate With n-butyllithium In tetrahydrofuran; hexane at -20℃; for 0.75h; Metallation; Stage #2: chloro-diphenylphosphine In tetrahydrofuran; hexane at -78℃; for 0.166667h; Substitution; | 89% |
| Stage #1: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate With n-butyllithium In hexane; dichloromethane at -78℃; for 1h; Stage #2: chloro-diphenylphosphine In hexane; dichloromethane at -78 - 20℃; | 88% |
| With n-butyllithium In hexane; dichloromethane at -78℃; for 1.08333h; | 84% |

-
-
893-33-4
4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedione

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| With NaOH In ethanol; water (N2) soln. 1M NaOH was added to soln. ligand in EtOH at room temp., EuCl3*6H2O was added, stirred for 4 h at 50°C, evapd., residue was washed with hexane, dissolved in water, soln. (C4mim)Br in aq. EtOH was added, stirred for 1 h at room temp.; ppt. was filtered, washed with hexane, dried under vac.; elem. anal.; | 77% |

-
-
75-15-0
carbon disulfide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.25h; Inert atmosphere; Stage #2: carbon disulfide In tetrahydrofuran at 20℃; for 0.5h; Mechanism; Inert atmosphere; | 76% |

-
-
123-54-6
acetylacetone

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| Stage #1: acetylacetone With sodium hydroxide In ethanol; water pH=8; Stage #2: 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate In ethanol; water at 50℃; for 0.5h; Stage #3: terbium(III) nitrate pentahydrate In ethanol; water at 50℃; for 2h; | 72% |

-
-
124-38-9
carbon dioxide

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
-
671779-18-3
N-methyl-N'-n-butylimidazolium-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 100℃; under 60006 Torr; for 12h; Autoclave; | 70% |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In acetonitrile Inert atmosphere; Glovebox; Schlenk technique; | 41% |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
13463-40-6, 71564-23-3
iron pentacarbonyl

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: CO; Sonication; Fe complex and ligand dispersed in ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate, conicated at 40-50°C for 30 min (air); pptd.(ethanol), elem. anal., XRD; | 28% |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
-
366491-14-7
1-n-butyl-3-methyl-2,3-dihydro-imidazol-2-ylidene

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane; dichloromethane at -78℃; for 1h; | |
| With N,N,N,N-tetraethylammonium tetrafluoroborate In N,N-dimethyl-formamide at 25℃; Inert atmosphere; Electrolysis; | |
| In N,N-dimethyl-formamide at 25℃; Inert atmosphere; Electrolysis; |
-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
A
-
616-47-7
1-methyl-1H-imidazole

-
B
-
106-98-9
1-butylene

-
C
-
2366-52-1
1-fluorobutane

| Conditions | Yield |
|---|---|
| With cesium fluoride at 150℃; for 96h; |

-
-
98-01-1
furfural

-
-
292638-85-8
acrylic acid methyl ester

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

-
A
-
87102-10-1
methyl 2-(furan-2-yl(hydroxy)methyl)acrylate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane at 20℃; for 24h; Baylis-Hillman; |

-
-
174501-64-5
3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In water; acetonitrile Radiolysis; |

1-Butyl-3-methylimidazolium hexafluorophosphate Specification
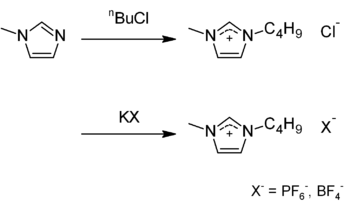
The 1-Butyl-3-methylimidazolium hexafluorophosphate, with the CAS registry number 174501-64-5, is also known as 3-Butyl-1-methylimidazolium hexafluorophosphate. It belongs to the product categories of Imidazolium Compounds; Imidazolium Salts (Ionic Liquids); Ionic Liquids; Synthetic Organic Chemistry; Ionic liquid. This chemical's molecular formula is C8H15N2.PF6 and molecular weight is 284.18. What's more, its systematic name is 1-Butyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate. This chemical is a viscous, colourless, hydrophobic and non-water soluble ionic liquid. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water.
Preparation: this chemical can be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is harmful if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: CCCCn1cc[n+](c1)C.F[P-](F)(F)(F)(F)F
(2)Std. InChI: InChI=1S/C8H15N2.F6P/c1-3-4-5-10-7-6-9(2)8-10;1-7(2,3,4,5)6/h6-8H,3-5H2,1-2H3;/q+1;-1
(3)Std. InChIKey: IXQYBUDWDLYNMA-UHFFFAOYSA-N
Related Products
- 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
- 1-Butyl-3-methylimidazolium bromide
- 1-Butyl-3-methylimidazolium chloride
- 1-Butyl-3-methylimidazolium dicyanamide
- 1-Butyl-3-methylimidazolium hexafluoroan
- 1-Butyl-3-methylimidazolium hexafluorophosphate
- 1-Butyl-3-methylimidazolium hydrogen carbonate solution
- 1-Butyl-3-methylimidazolium hydrogensulfate
- 1-Butyl-3-methylimidazolium iodide
- 1-Butyl-3-methylimidazolium methanesulfonate
- 174501-65-6
- 17450-34-9
- 17450-56-5
- 1745-05-7
- 17450-68-9
- 1745-07-9
- 174508-31-7
- 174514-06-8
- 174514-07-9
- 17451-61-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View