-
Name
1-CHLORO-2-METHYLBUTANE
- EINECS 210-466-9
- CAS No. 616-13-7
- Density 0.867 g/cm3
- Solubility
- Melting Point -104 °C
- Formula C5H11Cl
- Boiling Point 98.3 °C at 760 mmHg
- Molecular Weight 106.595
- Flash Point 9.8 °C
- Transport Information UN 1107 3/PG 2
- Appearance
- Safety 16-33
- Risk Codes 11
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms 1-Chloro-2-methylbutane;2-Methylbutyl chloride;
- PSA 0.00000
- LogP 2.27130
1-Chloro-2-methylbutane Specification
The CAS register number of 1-Chloro-2-methylbutane is 616-13-7. It also can be called as Butane,1-chloro-2-methyl- and the IUPAC name about this chemical is 1-chloro-2-methylbutane. The molecular formula about this chemical is C5H11Cl and the molecular weight is 106.59. It belongs to the following product categories, such as Alkyl; Halogenated Hydrocarbons; Organic Building Blocks and so on. This chemical is highly flammable. When you are using it, please keep away from sources of ignition and take precautionary measures against static discharges.
Physical properties about 1-Chloro-2-methylbutane are: (1)ACD/LogP: 2.91; (2)ACD/LogD (pH 5.5): 2.91; (3)ACD/LogD (pH 7.4): 2.91; (4)ACD/BCF (pH 5.5): 95.96; (5)ACD/BCF (pH 7.4): 95.96; (6)ACD/KOC (pH 5.5): 912.8; (7)ACD/KOC (pH 7.4): 912.8; (8)#Freely Rotating Bonds: 2; (9)Index of Refraction: 1.403; (10)Molar Refractivity: 30.02 cm3; (11)Molar Volume: 122.8 cm3; (12)Polarizability: 11.9x10-24cm3; (13)Surface Tension: 22.2 dyne/cm; (14)Enthalpy of Vaporization: 32.37 kJ/mol; (15)Boiling Point: 98.3 °C at 760 mmHg; (16)Vapour Pressure: 46.2 mmHg at 25°C.
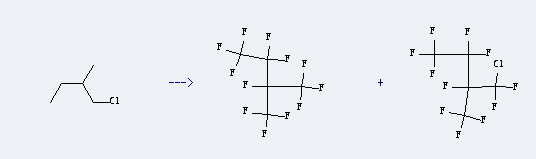
Uses of 1-Chloro-2-methylbutane: it can be used to produce 1,1,1,2,2,3,4,4,4-nonafluoro-3-trifluoromethyl-butane and 1-chloro-1,1,2,3,3,4,4,4-octafluoro-2-trifluoromethyl-butane at temperature of -35 - 0 ℃. This reaction will need reagent Fluorine with reaction time of 1.5 hours. The yield is about 39%. This reaction needs other conditions like irradiation//steady-state flow process; the hydrocarbon vapor is condensed onto a NaF preaerosol and then subjected to attack by an excess of elemental F in He at first in the dark, then under UV irra.
You can still convert the following datas into molecular structure:
(1)SMILES: ClCC(C)CC
(2)InChI: InChI=1/C5H11Cl/c1-3-5(2)4-6/h5H,3-4H2,1-2H3
(3)InChIKey: IWAKWOFEHSYKSI-UHFFFAOYAH
(4)Std. InChI: InChI=1S/C5H11Cl/c1-3-5(2)4-6/h5H,3-4H2,1-2H3
(5)Std. InChIKey: IWAKWOFEHSYKSI-UHFFFAOYSA-N
Related Products
- 1-Chloro-2-methylbutane
- 6161-50-8
- 616-16-0
- 6161-62-2
- 6161-65-5
- 61617-00-3
- 61618-27-7
- 6161-83-7
- 616201-80-0
- 616202-92-7
- 616204-22-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View