-
Name
1-Deoxynojirimycin
- EINECS 606-239-2
- CAS No. 19130-96-2
- Article Data102
- CAS DataBase
- Density 1.456 g/cm3
- Solubility soluble in water
- Melting Point 195-196 °C
- Formula C6H13NO4
- Boiling Point 361.1 °C at 760 mmHg
- Molecular Weight 163.174
- Flash Point 197.3 °C
- Transport Information
- Appearance white crystalline solid
- Safety 24/25
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms UNII-FZ56898FLE;1,5-Deoxy-1,5-imino-D-mannitol;
- PSA 92.95000
- LogP -2.63800
Synthetic route
-
-
69567-11-9, 76738-52-8, 126836-30-4
2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 20% palladium hydroxide-activated charcoal; hydrogen In methanol; water for 14h; | 100% |
| With hydrogenchloride; palladium 10% on activated carbon; hydrogen In ethanol; water at 20℃; under 3000.3 Torr; for 20h; pH=Ca. 2; | 93% |
| Stage #1: 2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol With hydrogenchloride In ethanol for 0.25h; pH=Ca. 2; Inert atmosphere; Stage #2: With palladium 10% on activated carbon; hydrogen In ethanol under 3000.3 Torr; for 20h; Inert atmosphere; | 93% |
-
-
157363-86-5
N-benzyl-3,4-di-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In acetic acid for 15h; | 100% |
| With hydrogen; acetic acid; palladium for 18h; | 100% |
| With palladium 10% on activated carbon; hydrogen In acetic acid at 20℃; for 19h; | 98% |
-
-
1190302-84-1
(2R,4aR,7S,8R,8aR)-tert-butyl 8-hydroxy-7-(methoxymethyl)-2-phenyltetrahydro-4H-[1,3]dioxino[5,4-b]pyridine-5(4aH)-carboxylate

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 18h; Reflux; Inert atmosphere; | 100% |
-
-
132198-14-2
N-Benzhydryl-1-deoxynojirimycin

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In methanol under 3102.9 Torr; for 24h; | 99% |
| With hydrogen; palladium dihydroxide | 90% |
| With hydrogen; palladium on activated charcoal In methanol | 50% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In tetrahydrofuran; water at 20℃; under 760.051 Torr; for 48h; | 98% |
| With hydrogen; palladium dihydroxide In methanol for 24h; |
-
-
15218-38-9
nojirimycin

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In ethanol; water under 750.06 Torr; for 18h; Ambient temperature; | 97% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 12h; Heating; | 94% |
-
-
1017587-22-2
(2R,3R,4R,5S)-(3,4-dibenzyloxy-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinyl)methanol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In ethanol at 20℃; for 48h; | 92% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In acetic acid; tert-butyl alcohol under 3750.3 Torr; for 48h; Ambient temperature; | 91% |
-
-
149302-60-3
N-bentyl-2,3,4,6-tetra-O-benzyl-1-deoxynojirimycin

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With 10 wt% Pd(OH)2 on carbon; hydrogen In ethanol; chloroform at 20℃; for 20h; | 91% |
-
-
108818-06-0
(2R,3R,4S,5S)-4,5-(isopropylenedioxy)-3-(methoxymethoxy)-2-<(methoxymethoxy)methyl>piperidine

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 1h; Heating; | 90% |
-
-
107025-38-7
(3aS,6R,7R,7aS)-6-(tert-Butyl-dimethyl-silanyloxymethyl)-2,2-dimethyl-hexahydro-[1,3]dioxolo[4,5-c]pyridin-7-ol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid Ambient temperature; | 90% |
-
-
72458-46-9
N-benzyl-1-deoxynojirimycin

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | 89% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With 10 wt% Pd(OH)2 on carbon; hydrogen; potassium carbonate In water under 2585.81 Torr; | 80% |
-
-
123149-59-7, 132152-32-0
Nojirimycin bisulfite

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With barium dihydroxide; hydrogen; nickel In water | 75% |
-
-
75016-28-3
((2S,3R,4S)-2,3,4,6-Tetrahydroxy-5-oxo-hexyl)-carbamic acid benzyl ester

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; potassium carbonate; palladium on activated charcoal In methanol; water at 60℃; under 60004.8 Torr; for 2h; | 75% |
| With palladium 10% on activated carbon; hydrogen In methanol; water at 20℃; under 2585.81 Torr; for 24h; | 717 mg |
-
-
1427467-92-2
(2R,3R,4R,5S)-3-benzyloxy-2-benzyloxymethyl-4,5-isopropylidenedioxypiperidine

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium 10% on activated carbon; water; hydrogen In methanol; ethyl acetate at 20℃; for 48h; | 75% |
-
-
74244-24-9
6-Amino-6-desoxy-L-sorbofuranose-hydrochlorid

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; hydrogen at 20℃; under 3750.38 Torr; for 16h; Inert atmosphere; | 70% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With 20 % Pd(OH)2/C; ammonium acetate; hydrogen In methanol; water at 20℃; for 24h; | 68% |
-
-
223421-29-2
(+)-(3aR,5RS,6R,6aS)-3a,5,6,6a-tetrahydro-5,6-dihydroxyfuro<2,3-d>isoxazole-3-methanol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 25℃; under 750.06 Torr; for 15h; | 65% |
-
-
146897-25-8
5-azido-5-deoxy-D-glucofuranose

-
A
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
B
-
73285-50-4, 73465-43-7, 75172-81-5, 118464-54-3, 126663-84-1, 126923-63-5, 136658-34-9
1-deoxynojirimycin hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 5-azido-5-deoxy-D-glucofuranose With palladium 10% on activated carbon; hydrogen In water at 20℃; under 2068.65 - 2585.81 Torr; Stage #2: With hydrogenchloride In water pH=2; | A 16% B 56% |

-
A
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium dihydroxide | A 55% B 21% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In 1,4-dioxane; water for 24h; Ambient temperature; | 54% |
-
-
81703-56-2
5-amino-5-deoxy-D-glucose-1-sulfonic acid

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With barium hydroxide octahydrate; hydrogen; Raney-Ni (W-4) In water under 760 Torr; for 8h; Ambient temperature; | 53% |
-
-
1051942-01-8
(2R,3R,4S,5S)-5-(Benzyloxy)-2-(hydroxymethyl)piperidine-3,4-diol

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; | 53% |
-
-
768387-39-9
(1R,3aR,7aR,8R)-6,6-dimethylhexahydro-1,5,7-trioxa-3-azacyclopropa[a]naphthalene-3-carboxylic acid tert-butyl ester

-
A
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| Stage #1: (1R,3aR,7aR,8R)-6,6-dimethylhexahydro-1,5,7-trioxa-3-azacyclopropa[a]naphthalene-3-carboxylic acid tert-butyl ester With potassium hydroxide In 1,4-dioxane; water Heating; Stage #2: With hydrogenchloride In methanol; water at 60℃; for 1h; Stage #3: With DOWEX-1X2 (OH(1-) form) In water Further stages.; | A 33% B 51% |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In ethanol under 25857.4 Torr; for 24h; | 19% |
-
-
127708-83-2
3,6-di-O-benzyl-5-azido-5-deoxy-α/β-D-glucofuranose

-
A
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium hydroxide - carbon In methanol; water; acetic acid under 3800 Torr; for 48h; Ambient temperature; Yield given; | A n/a B 5% |
| With hydrogen; palladium hydroxide - carbon In methanol; water; acetic acid under 3800 Torr; for 48h; Ambient temperature; Yields of byproduct given; | A n/a B 5% |
-
-
57-04-5
dihydroxyacetone phosphate

-
-
114395-07-2
(RS)-3-azido-2-hydroxypropanal

-
A
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

| Conditions | Yield |
|---|---|
| Multistep reaction; |

-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
958880-39-2
C12H15N5O3

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol at 20℃; for 14h; | 100% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
73285-50-4, 73465-43-7, 75172-81-5, 118464-54-3, 126663-84-1, 126923-63-5, 136658-34-9
1-deoxynojirimycin hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water | 100% |
| With hydrogenchloride In water at 10℃; pH=2; Molecular sieve; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; Inert atmosphere; | 99% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-44-7
C13H18O2

-
-
1383152-03-1
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(m-tolyloxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H18O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 98% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383152-78-0
C12H16O2

-
-
1383152-95-1
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(3-(2-propoxyphenyl)propyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C12H16O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen for 24h; | 97% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383152-81-5
C12H16O2

-
-
1383152-98-4
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(3-(3-propoxyphenyl)propyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C12H16O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 97% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1057671-19-8
C12H16O2

-
-
1383153-01-2
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(3-(4-propoxyphenyl)propyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C12H16O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 97% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
91476-10-7
3-bromoprop-1-ene-2-phosphonic acid diethyl ester

-
-
165805-33-4
[1-((2R,3R,4R,5S)-3,4,5-Trihydroxy-2-hydroxymethyl-piperidin-1-ylmethyl)-vinyl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetone at 25℃; for 3h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-58-3
C12H15FO2

-
-
1383152-21-3
(2R,3R,4R,5S)-1-(6-(2-fluorophenoxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C12H15FO2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon In ethanol for 24h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1369796-75-7
6-(3-fluorophenoxy)hexanal

-
-
1383152-24-6
(2R,3R,4R,5S)-1-(6-(3-fluorophenoxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: 6-(3-fluorophenoxy)hexanal With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
289032-98-0
C12H15FO2

-
-
1383152-27-9
(2R,3R,4R,5S)-1-(6-(4-fluorophenoxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C12H15FO2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-81-2
C13H15F3O2

-
-
1383152-45-1
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-(trifluoromethyl)phenoxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H15F3O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383152-89-3
C15H20O2

-
-
1383153-07-8
(2R,3R,4R,5S)-1-(3-(3-(cyclohexyloxy)phenyl)propyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C15H20O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 20h; | 96% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-49-2
C16H24O2

-
-
1383152-12-2
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(2-isopropyl-5-methylphenoxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C16H24O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 95% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383152-92-8
C15H20O2

-
-
1383153-10-3
(2R,3R,4R,5S)-1-(3-(4-(cyclohexyloxy)phenyl)propyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C15H20O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 95% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-78-7
C13H15F3O2

-
-
1383152-42-8
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(3-(trifluoromethyl)phenoxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H15F3O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 94% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-84-5
C13H15F3O3

-
-
1383152-48-4
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-(trifluoromethoxy)phenoxy)hexyl) piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H15F3O3 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol; water under 2585.7 Torr; for 36h; | 93% |
| With formic acid In water for 20h; Heating; Yield given; | |
| With formic acid at 80℃; for 3h; |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-75-4
C13H15F3O2

-
-
1383152-39-3
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(2-(trifluoromethyl)phenoxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H15F3O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 93% |
| With sodium cyanoborohydride In methanol |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383151-90-3
C13H18O3

-
-
1383152-54-2
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-methoxyphenoxy)hexyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H18O3 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 93% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383149-90-3
C13H26O2

-
-
1383150-05-7
(2R,3R,4R,5S)-1-(6-(heptan-4-yloxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H26O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon In ethanol for 24h; | 93% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383153-50-1
C14H26O2

-
-
1383150-14-8
(2R,3R,4R,5S)-1-(6-((2-ethylcyclohexyl)oxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C14H26O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 93% |
-
-
19130-96-2
1,5-dideoxy-1,5-imino-D-glucitol

-
-
1383153-78-3
C13H24O2

-
-
1383150-37-5
(2R,3R,4R,5S)-1-(6-(cycloheptyloxy)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol With acetic acid at 20℃; Stage #2: C13H24O2 With acetic acid In ethanol at 20℃; for 1h; Inert atmosphere; Stage #3: With palladium 10% on activated carbon; hydrogen In ethanol for 24h; | 93% |
1-Deoxynojirimycin Chemical Properties
Molecular structure of 1-Deoxynojirimycin (CAS NO.19130-96-2) is:
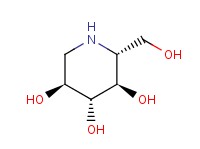
Product Name: 1-Deoxynojirimycin
CAS Registry Number: 19130-96-2
IUPAC Name: (2R,3R,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-triol
Molecular Weight: 163.17172 [g/mol]
Molecular Formula: C6H13NO4
XLogP3-AA: -2.3
H-Bond Donor: 5
H-Bond Acceptor: 5
Flash Point: 163.7 °C
Enthalpy of Vaporization: 64.97 kJ/mol
Boiling Point: 347 °C at 760 mmHg
Vapour Pressure: 9.72E-06 mmHg at 25°C
Melting Point: 195-196 °C
Product Categories: Miscellaneous Natural Products; All Inhibitors; Glycosidase Inhibitors; Inhibitors; Miscellaneous Enzyme
1-Deoxynojirimycin Uses
1-Deoxynojirimycin (CAS NO.19130-96-2) is an alpha-glucosidase inhibitor with antiviral action.
1-Deoxynojirimycin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED SKIN AND APPENDAGES (SKIN): HAIR: OTHER | International Journal of Toxicology. Vol. 16(Suppl, |
1-Deoxynojirimycin Specification
1-Deoxynojirimycin , its cas register number is 19130-96-2. It also can be called 1,5-Deoxy-1,5-imino-D-mannitol ; 1-Deoxymannojirimycin ; UNII-FZ56898FLE ; 3,4,5-Piperidinetriol, 2-(hydroxymethyl)-, (2R-(2alpha,3beta,4alpha,5beta))- .It is a white crystalline solid.
Related Products
- 1-Deoxynojirimycin
- 1913-12-8
- 19131-99-8
- 19132-06-0
- 191327-28-3
- 191327-30-7
- 19132-91-3
- 191330-56-0
- 191338-86-0
- 19134-50-0
- 191348-14-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View