-
Name
1-Phenoxy-2-propanol
- EINECS 212-222-7
- CAS No. 770-35-4
- Article Data68
- CAS DataBase
- Density 1.054 g/cm3
- Solubility 15.1g/L at 20℃
- Melting Point 11 °C
- Formula C9H12O2
- Boiling Point 249.303 °C at 760 mmHg
- Molecular Weight 152.193
- Flash Point 104.417 °C
- Transport Information
- Appearance colourless liquid
- Safety 23-26
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Phenoxy-1-methylethanol;NSC 24015;Phenoxyisopropanol;Propylene phenoxetol;rac-1-Phenoxy-2-propanol;b-Phenoxyisopropanol;2-Propanol, 1-phenoxy-;
- PSA 29.46000
- LogP 1.44620
Synthetic route

| Conditions | Yield |
|---|---|
| With formic acid; C33H33ClIrNO; sodium formate In water at 80℃; for 14h; pH=4.5; Catalytic behavior; Reagent/catalyst; Inert atmosphere; chemoselective reaction; | 98% |
| With formic acid; C18H24ClIrN3 In water at 80℃; for 12h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 96% |
| With [RhCl2(p-cymene)]2; dimethylamine borane In tetrahydrofuran at 70℃; for 24h; Inert atmosphere; Sealed ampoule; | 94% |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate; hydrogen In toluene at 75℃; under 37503.8 Torr; for 24h; regioselective reaction; | 98% |
| With hydrogen; Pd/magnetite In ethyl acetate at 23℃; under 760.051 Torr; for 0.5h; | 97% |
| With Bu3Sn(HMPA)I; tri-n-butyl-tin hydride In tetrahydrofuran at 60℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| With Al2O3/MgO composite at 120℃; for 5h; Inert atmosphere; | 97.5% |
| With sodium hydroxide | |
| With sodium hydroxide at 150℃; | |
| With Methyltriphenylphosphonium bromide at 80 - 110℃; under 7500.75 Torr; for 8h; Temperature; Reagent/catalyst; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol | 93% |
-
-
75-56-9, 16033-71-9
methyloxirane

-
-
108-95-2
phenol

-
A
-
770-35-4
1-phenoxy-2-propanol

-
B
-
4169-04-4
2-phenoxy-1-propanol

| Conditions | Yield |
|---|---|
| at 116.9℃; | A 92% B 8% |
| With boron trifluoride diethyl etherate at 76.9℃; Yield given. Yields of byproduct given; | |
| With BiCl6(3-)*2C4H10N2*ClH*3H(1+)*H2O In neat (no solvent) at 20℃; for 0.166667h; Overall yield = 91 %; |


| Conditions | Yield |
|---|---|
| With hydrogen; triethylamine; palladium on activated charcoal In methanol under 760 Torr; for 24h; Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| With magnesium-aluminum hydrotalcite completely intercalated on silicate4 at 150℃; for 10h; Reagent/catalyst; Temperature; | A n/a B 85% |

-
-
122-60-1
Phenyl glycidyl ether

-
-
98-95-3
nitrobenzene

-
A
-
770-35-4
1-phenoxy-2-propanol

-
B
-
16112-55-3, 113279-34-8
N-(2-hydroxy-3-phenoxypropyl)aniline

-
C
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In ethanol Reflux; | A 6% B 21% C 73% |


| Conditions | Yield |
|---|---|
| at 160℃; |

| Conditions | Yield |
|---|---|
| With sodium ethanolate |
-
-
139-02-6
sodium phenoxide

-
-
75-56-9, 16033-71-9
methyloxirane

-
-
108-95-2
phenol

-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With benzene |


| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| With phenol In benzene |

| Conditions | Yield |
|---|---|
| aluminium trichloride |
-
-
57-55-6
propylene glycol

-
-
28899-97-0
triphenylbismuth(V) diacetate

-
A
-
770-35-4
1-phenoxy-2-propanol

-
B
-
4169-04-4
2-phenoxy-1-propanol

| Conditions | Yield |
|---|---|
| In dichloromethane for 4h; Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| In dichloromethane for 5h; Heating; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With nickel boride Ambient temperature; Yield given; |
-
-
75-56-9, 16033-71-9
methyloxirane

-
-
108-95-2
phenol

-
A
-
770-35-4
1-phenoxy-2-propanol

-
C
-
126797-90-8
(2-Hydroxy-propyl)-phosphinic acid 2-(2-methoxyphosphinoyl-1-methyl-ethoxyphosphinoyl)-1-methyl-ethyl ester

| Conditions | Yield |
|---|---|
| With phosphorous; triethylamine |


| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 370 K, 2.) 395-400 K; Multistep reaction. Further byproducts given; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 370 K, 2.) 395-400 K; Multistep reaction. Further byproducts given; |

-
-
174309-44-5
Butyric acid (R)-1-methyl-2-phenoxy-ethyl ester

-
A
-
6065-72-1
2-chloroethyl butanoate

-
B
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| In hexane at 30℃; Equilibrium constant; lipase B from Candida antarctica; |

| Conditions | Yield |
|---|---|
| With benzenesulfonic acid at 100℃; |


| Conditions | Yield |
|---|---|
| anion exchange resin A (Cl-type) at 100℃; for 12h; | A 94.4 %Chromat. B 5.3 %Chromat. |


| Conditions | Yield |
|---|---|
| In methanol |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water for 8h; |
-
-
57-55-6
propylene glycol

-
-
107-21-1
ethylene glycol

-
-
108-95-2
phenol

-
A
-
122-99-6
2-Phenoxyethanol

-
B
-
770-35-4
1-phenoxy-2-propanol

-
C
-
4169-04-4
2-phenoxy-1-propanol

| Conditions | Yield |
|---|---|
| With sodium carbonate; urea; zinc(II) oxide at 175℃; |

-
-
57-55-6
propylene glycol

-
-
108-95-2
phenol

-
A
-
770-35-4
1-phenoxy-2-propanol

-
B
-
4169-04-4
2-phenoxy-1-propanol

| Conditions | Yield |
|---|---|
| With sodium carbonate; urea; zinc(II) oxide at 170 - 190℃; | |
| With potassium carbonate; Diethyl carbonate at 130℃; for 10h; | A 73 %Spectr. B 7 %Spectr. |

-
-
57-55-6
propylene glycol

-
-
108-94-1
cyclohexanone

-
A
-
770-35-4
1-phenoxy-2-propanol

-
B
-
4169-04-4
2-phenoxy-1-propanol

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon In neat (no solvent) at 130℃; for 60h; Inert atmosphere; Green chemistry; Overall yield = 40 %; Overall yield = 44 %Spectr.; | |
| With 5%-palladium/activated carbon at 130℃; for 60h; Inert atmosphere; Overall yield = 40 %; Overall yield = 44 %Spectr.; |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide Alkaline conditions; |

-
-
1746-13-0
allyl phenyl ether

-
A
-
6180-61-6
3-phenoxypropanol

-
B
-
538-43-2
1-phenoxy-2,3-propanediol

-
C
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: bis(cyclopentadienyl)titanium dichloride; magnesium / tetrahydrofuran / 0 °C / Inert atmosphere 2: dihydrogen peroxide / Alkaline conditions View Scheme |


| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate In dichloromethane for 48h; Inert atmosphere; | 97% |
| With 4-acetamido-2,2,6,6-tetramethylpiperidin-1-oxoammonium nitrate; silica gel In dichloromethane at 20℃; for 52h; | 95% |
| With sodium hypochlorite pentahydrate; 4-acetamido-2,2,6,6-tetramethylpiperidine-N-hydroxyammonium tetrafluoroborate In water; acetonitrile at 20℃; for 2h; | 65% |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 110 - 240℃; under 7.50075 - 75.0075 Torr; for 6.5h; | 93% |
-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With bismuth(III) chloride at 25 - 26℃; for 0.166667h; Stage #2: 1-benzylspiro[indoline-3,2′-oxiran]-2-one at 25 - 26℃; for 12h; regioselective reaction; | 90% |
-
-
876-27-7
4-chlorophenyl acetate

-
-
770-35-4
1-phenoxy-2-propanol

-
-
151636-14-5
Acetic acid (R)-1-methyl-2-phenoxy-ethyl ester

| Conditions | Yield |
|---|---|
| With Novozym 435; In toluene at 70℃; for 46h; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: phenyl isothiocyanate In tetrahydrofuran at 20℃; for 18h; Further stages.; | 88% |
-
-
906069-29-2
(R)-3-(3,3-Dimethyl-2-oxo-butyryl)-2,2-dimethyl-thiazolidine-4-carboxylic acid

-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With dmap; camphor-10-sulfonic acid; dicyclohexyl-carbodiimide In dichloromethane | 73.7% |

| Conditions | Yield |
|---|---|
| With [Ir(ppy)2(dtbpy)]PF6; sodium salt of dibutyl phosphate; Methyl thioglycolate In N,N-dimethyl acetamide at 25℃; for 72h; Sealed tube; Irradiation; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 48h; Inert atmosphere; | 69% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: p-nitrophenyl isothiocyanate In tetrahydrofuran at 20℃; for 18h; Further stages.; | 68% |
-
-
906069-48-5
(R)-2,2-Dimethyl-3-[2-oxo-2-(2,4,6-trimethyl-phenyl)-acetyl]-thiazolidine-4-carboxylic acid

-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With dmap; camphor-10-sulfonic acid; dicyclohexyl-carbodiimide In dichloromethane | 67.4% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 0℃; Stage #2: phenyl isothiocyanate In tetrahydrofuran at 20℃; for 0.333333h; Stage #3: propoxycarbonyl chloride In tetrahydrofuran at 60℃; for 3h; Further stages.; | 67% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; 3-mercaptopropionic acid at 65℃; for 5h; | 66.4% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 0℃; Stage #2: phenyl isothiocyanate In tetrahydrofuran at 20℃; for 0.333333h; Stage #3: phenyl chloroformate In tetrahydrofuran at 60℃; for 3h; Further stages.; | 64% |

-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; triphenylphosphine In dichloromethane at 0 - 20℃; | 63% |
-
-
770-35-4
1-phenoxy-2-propanol

-
-
103-72-0
phenyl isothiocyanate

-
-
592-34-7
carbonochloridic acid, butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 0℃; Stage #2: phenyl isothiocyanate In tetrahydrofuran at 20℃; for 0.333333h; Stage #3: carbonochloridic acid, butyl ester In tetrahydrofuran at 60℃; for 3h; Further stages.; | 56% |

-
-
770-35-4
1-phenoxy-2-propanol

-
-
35205-54-0
1-methyl-2-phenoxyethylamine

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol In dichloromethane cooling; Stage #2: With dibenzyl azodicarboxylate In dichloromethane cooling; Stage #3: With trifluoroacetic acid In dichloromethane Further stages.; | 55% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: 1-isothiocyanato-4-methylbenzene In tetrahydrofuran at 20℃; for 18h; Further stages.; | 55% |
-
-
31643-49-9
4-Nitrophthalonitrile

-
-
770-35-4
1-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 98h; | 48% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenoxy-2-propanol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: 4-Methoxyphenyl isothiocyanate In tetrahydrofuran at 20℃; for 18h; Further stages.; | 42% |
1-Phenoxy-2-propanol Specification
1-Phenoxy-2-propanol is an organic compound with the formula C9H12O2, and its systematic name is the same with the product name. With the CAS registry number 770-35-4, it is also named as 2-Phenoxy-1-methylethanol. It belongs to the product category of Benzhydrols, Benzyl & Special Alcohols. Its EINECS number is 212-222-7. In addition, the molecular weight is 152.19. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides. It is used as intermediate of phenoxybenzamine.
Physical properties of 1-Phenoxy-2-propanol are: (1)ACD/LogP: 1.601; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.60; (4)ACD/LogD (pH 7.4): 1.60; (5)ACD/BCF (pH 5.5): 9.70; (6)ACD/BCF (pH 7.4): 9.70; (7)ACD/KOC (pH 5.5): 176.98; (8)ACD/KOC (pH 7.4): 176.98; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 29.46 Å2; (13)Index of Refraction: 1.517; (14)Molar Refractivity: 43.691 cm3; (15)Molar Volume: 144.332 cm3; (16)Polarizability: 17.32×10-24cm3; (17)Surface Tension: 37.69 dyne/cm; (18)Density: 1.054 g/cm3; (19)Flash Point: 104.417 °C; (20)Enthalpy of Vaporization: 51.409 kJ/mol; (21)Boiling Point: 249.303 °C at 760 mmHg; (22)Vapour Pressure: 0.012 mmHg at 25°C.
Preparation: this chemical can be prepared by phenoxymethyl-oxirane by heating. This reaction will need reagents NaI, n-Bu3SnH, AIBN and solvent 1,2-dimethoxy-ethane with the reaction time of 2 hours. The yield is about 91%.

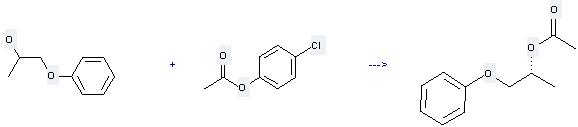
Uses of 1-Phenoxy-2-propanol: it can be used to produce acetic acid 1-methyl-2-phenoxy-ethyl ester at the temperature of 70 °C. It will need reagents (m-H)(C4Ph4COHOCC4Ph4)>, Novozym 435 and solvent toluene with the reaction time of 46 hours. The yield is about 88%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes. You should not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer). In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O(c1ccccc1)CC(O)C
(2)Std. InChI: InChI=1S/C9H12O2/c1-8(10)7-11-9-5-3-2-4-6-9/h2-6,8,10H,7H2,1H3
(3)Std. InChIKey: IBLKWZIFZMJLFL-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 2gm/kg (2000mg/kg) | National Technical Information Service. Vol. OTS0539745. | |
| rat | LD50 | oral | 2830mg/kg (2830mg/kg) | National Technical Information Service. Vol. OTS0539745. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View