-
Name
2-(2-Methoxyphenoxy)ethylamine
- EINECS 606-000-2
- CAS No. 1836-62-0
- Article Data28
- CAS DataBase
- Density 1.06 g/cm3
- Solubility
- Melting Point
- Formula C9H13NO2
- Boiling Point 261.1 °C at 760 mmHg
- Molecular Weight 167.208
- Flash Point 120.7 °C
- Transport Information UN 2735
- Appearance Colorless or slightly yellowish liquid
- Safety
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ethylamine,2-(o-methoxyphenoxy)- (7CI,8CI);2-(o-Methoxyphenoxy)ethylamine;Guaiacoxyethylamine;
- PSA 44.48000
- LogP 1.73300
Synthetic route

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 21h; Reflux; | 100% |
| Stage #1: N-[2-(2-methoxyphenoxy)ethyl]acetamide With hydrogenchloride; water for 4h; Heating / reflux; Stage #2: With sodium hydroxide In water pH=8 - 9; | 74.8% |
-
-
64464-07-9
2-[(2-methoxy)phenoxy]ethylamine hydrochloride

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0℃; pH=12 - 14; | 97% |
-
-
26646-63-9
2-[2-(2-methoxyphenoxy)ethyl]-1H-isoindole-1,3(2H)-dione

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 110℃; for 7h; | 91.2% |
| With hydrazine hydrate In water at 20℃; for 5h; Gabriel synthesis; | 86% |
| With hydrazine hydrate; acetic acid In methanol for 4h; Reflux; | 86% |
-
-
214778-48-0
2-(2-Methoxy-phenoxy)-ethyl-carbamic acid tert-butyl ester

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-Methoxy-phenoxy)-ethyl-carbamic acid tert-butyl ester With hydrogenchloride In 1,4-dioxane for 2h; Heating / reflux; Stage #2: With sodium hydroxide In water; ethyl acetate | 90% |
| With trifluoroacetic acid In dichloromethane | 88% |
-
-
183427-87-4
2-(2-methoxyphenoxy)acetamide

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex In tetrahydrofuran at 90℃; for 16h; Inert atmosphere; | 88% |
| With dimethylsulfide borane complex In diethylene glycol dimethyl ether at 90℃; for 18h; | 55% |
| With diethylene glycol dimethyl ether; diborane |
-
-
4463-59-6
2-(methoxyphenoxy)ethylbromide

-
A
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
B
-
3258-70-6
2-(2-methoxyphenoxy)-N-[2-(2-methoxyphenoxy)ethyl]ethanamine

| Conditions | Yield |
|---|---|
| With ammonia |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq. NaOH / 7 h / 100 °C 2: dimethylformamide / Heating 3: NH2NH2*H2O / ethanol / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: NaOEt / ethanol / 0.5 h / Heating 1.2: 83 percent / KI / ethanol / Heating 2.1: 55 percent / BH3*Me2S / bis-(2-methoxy-ethyl) ether / 18 h / 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: NaOH / H2O / 100 °C 2: dimethylformamide / 0.5 h 3: hydrazine hydrate / ethanol / 0.75 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide / Heating 2: NH2NH2*H2O / ethanol / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: dimethylformamide / 0.5 h 2: hydrazine hydrate / ethanol / 0.75 h / Heating View Scheme | |
| Stage #1: 2-(methoxyphenoxy)ethylbromide With 18-crown-6 ether; potassium phtalimide In N,N-dimethyl-formamide at 50℃; for 3h; Gabriel Amine Synthesis; Stage #2: With methylamine In water at 50℃; for 2h; Gabriel Amine Synthesis; Stage #3: With sodium hydroxide In water at 20℃; for 1h; Gabriel Amine Synthesis; | |
| Multi-step reaction with 2 steps 1.1: 18-crown-6 ether / N,N-dimethyl-formamide / 3 h / 50 °C 2.1: methylamine / water / 2 h / 50 °C 2.2: 1 h / 20 °C View Scheme |
-
-
136918-14-4
phthalimide

-
-
53815-60-4
1-(2-chloroethoxy)-2-methoxybenzene

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 130℃; for 13h; |
-
-
53815-60-4
1-(2-chloroethoxy)-2-methoxybenzene

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrabutylammomium bromide / 180 - 185 °C 2: water; potassium hydroxide / 13 h / 130 °C View Scheme |
-
-
18181-71-0
2-(2-methoxyphenoxy)ethanol

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: thionyl chloride / dichloromethane / 2 h / 0 - 30 °C 2: N,N-dimethyl-formamide / 3 h / 170 °C 3: sodium hydroxide / water / 7 h / 110 °C View Scheme |

| Conditions | Yield |
|---|---|
| In ethanol at 60 - 70℃; Addition; | 87% |
-
-
27866-06-4
(adamantan-1-ylmethoxy-methyl)-oxirane

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1479050-25-3
1-(adamantan-1-ylmethoxy)-3-{[2-(2-methoxyphenoxy)-ethyl]amino}-2-propanol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Reflux; | 87% |
-
-
2461-42-9
3-(1-naphthyloxy)-1,2-epoxypropane

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1479050-16-2
1-((2-(2-methoxyphenoxy)ethyl)amino)-3-(naphthalen-1-yloxy)propan-2-ol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Inert atmosphere; Reflux; | 86% |
| In isopropyl alcohol Reflux; | 38% |

| Conditions | Yield |
|---|---|
| In ethanol | 84.3% |
| In ethanol Solvent; Heating; Reflux; | 84.3% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
25888-01-1
1,2-dimethyl-5-hydroxy-1H-indole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane; N,N-dimethyl-formamide at 20℃; for 16h; | 83.2% |
-
-
1047632-41-6
4-(3-bromopropoxy)-9H-carbazole

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| In acetonitrile for 5h; Inert atmosphere; Reflux; | 83% |
| With potassium iodide In acetonitrile for 5h; Reflux; Inert atmosphere; | 73% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 19% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1204700-40-2
(S)-5-methoxy-4-(oxiran-2-yl methoxy)-1H-indole

-
-
1204700-46-8
(2S)-1-(5-methoxy-1H-indol-4-yloxy)-3-(2-(2-methoxyphenoxy)ethylamino)propan-2-ol

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 82% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1204700-41-3
(R)-5-methoxy-4-(oxiran-2-yl methoxy)-1H-indole

-
-
1204700-47-9
(2R)-1-(5-methoxy-1H-indol-4-yloxy)-3-(2-(2-methoxyphenoxy)ethylamino)propan-2-ol

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 82% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Inert atmosphere; Reflux; | 82% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
-
72956-09-3
carvedilol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Inert atmosphere; Reflux; | 81% |
| With N-ethyl-N,N-diisopropylamine In 1,2-dimethoxyethane at 80 - 85℃; | 36.26% |
| With sulfuric acid; potassium carbonate In isopropyl alcohol at 80℃; for 6h; |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
144-62-7
oxalic acid

-
-
51997-51-4
4-(2,3-epoxypropoxy)carbazole

-
-
72956-09-3
(+/-) 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethyl]amino-2-propanol oxalate

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-methoxy-phenoxy)-ethylamine; 4-(2,3-epoxypropoxy)carbazole In isopropyl alcohol at 70 - 85℃; for 1h; Stage #2: oxalic acid In isopropyl alcohol at 50 - 85℃; for 3h; Heating / reflux; | 80.95% |


| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Inert atmosphere; Reflux; | 78% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
144-62-7
oxalic acid

-
-
72956-09-3
(+/-) 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethyl]amino-2-propanol oxalate

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-methoxy-phenoxy)-ethylamine; 4-(2,3-epoxypropoxy)carbazole In chlorobenzene at 125℃; for 3h; Heating / reflux; Stage #2: oxalic acid In water; chlorobenzene at 60 - 70℃; for 1h; pH=2.5 - 2.7; | 77% |
| Stage #1: 2-(2-methoxy-phenoxy)-ethylamine; 4-(2,3-epoxypropoxy)carbazole In chlorobenzene at 125℃; for 3h; Heating / reflux; Stage #2: oxalic acid In Isopropyl acetate; water at 20 - 50℃; for 3h; pH=2 - 2.5; | 74.6% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| Stage #1: trans-5-phenyl-1,4-dioxane-2-carboxylic acid With chloroformic acid ethyl ester; triethylamine In chloroform at 0℃; for 0.5h; Stage #2: 2-(2-methoxy-phenoxy)-ethylamine In chloroform at 20℃; for 3h; | 77% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1204700-39-9
5-methoxy-4-(oxiran-2-yl methoxy)-1H-indole

-
-
1204700-45-7
(2RS)-1-(5-methoxy-1H-indol-4-yloxy)-3-(2-(2-methoxyphenoxy)ethylamino)propan-2-ol

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 77% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1204700-37-7
(S)-7-methoxy-4-(oxiran-2-ylmethoxy)-1H-indole

-
-
1204700-43-5
(2S)-1-(7-methoxy-1H-indol-4-yloxy)-3-(2-(2-methoxyphenoxy)ethylamino)-propan-2-ol

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 77% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
90402-61-2
Ethyl 2-<6-chloro-1,3-dimethyl-2,4(1H,3H)-dioxopyrimidine-5-yl>-2-acrylate

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 24h; Ambient temperature; | 76% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
244123-12-4
2-(2-(benzyloxy)phenoxy)acetic acid

-
-
1255188-26-1
2-(2-(benzyloxy)phenoxy)-N-(2-(2-methoxyphenoxy) ethyl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-(benzyloxy)phenoxy)acetic acid With chloroformic acid ethyl ester; triethylamine In chloroform at 0℃; for 0.5h; Stage #2: 2-(2-methoxy-phenoxy)-ethylamine In chloroform at 20℃; for 3h; | 76% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
95093-95-1
S-(+)-4-(oxiranylmethoxy)-9H-carbazole

-
-
95094-00-1, 72956-09-3, 95093-99-5, 107741-96-8
(-)-Carvedilol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 2h; Reflux; Inert atmosphere; | 76% |
| In isopropyl alcohol for 4h; Reflux; | 70% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 25℃; for 72h; Inert atmosphere; Schlenk technique; | 76% |

-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
1479050-01-5
1-{[2-(2-methoxyphenoxy)ethyl]amino}-3-(2,3,4,9-tetrahydro-1H-carbazol-6-yloxy)-2-propanol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Reflux; | 75% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| With potassium iodide In acetonitrile for 5h; Reflux; Inert atmosphere; | 75% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
120351-75-9
N-[4-(4-Chloro-1-cyano-1-isopropyl-butyl)-phenyl]-acetamide

-
-
120351-13-5
N-(4-{1-Cyano-1-isopropyl-4-[2-(2-methoxy-phenoxy)-ethylamino]-butyl}-phenyl)-acetamide

| Conditions | Yield |
|---|---|
| at 95℃; | 74% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

-
-
67579-92-4
3-methoxy-4-nitrobenzoic acid chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane | 74% |
-
-
1836-62-0
2-(2-methoxy-phenoxy)-ethylamine

| Conditions | Yield |
|---|---|
| Stage #1: cis-3-phenyl-1,4-dioxane-2-carboxylic acid With chloroformic acid ethyl ester; triethylamine In chloroform at 0℃; for 0.5h; Stage #2: 2-(2-methoxy-phenoxy)-ethylamine In chloroform at 20℃; for 3h; | 74% |
2-(2-Methoxyphenoxy)ethylamine Chemical Properties
IUPAC Name: 2-(2-Methoxyphenoxy)ethanamine
Following is the structure of Ethanamine,2-(2-methoxyphenoxy)- (CAS NO.1836-62-0):
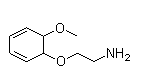
Empirical Formula: C9H13NO2
Molecular Weight: 167.205
Index of Refraction: 1.518
Molar Refractivity: 47.77 cm3
Molar Volume: 157.6 cm3
Density: 1.06 g/cm3
Flash Point: 120.7 °C
Surface Tension: 37.2 dyne/cm
Enthalpy of Vaporization: 49.88 kJ/mol
Boiling Point: 261.1 °C at 760 mmHg
Vapour Pressure of Ethanamine,2-(2-methoxyphenoxy)- (CAS NO.1836-62-0): 0.0118 mmHg at 25 °C
Product Categories of Ethanamine,2-(2-methoxyphenoxy)- (CAS NO.1836-62-0): Anilines, Aromatic Amines and Nitro Compounds; Amines; (intermediate of carvedilol); Carvedilol Intermediate
Canonical SMILES: COC1=CC=CC=C1OCCN
InChI: InChI=1S/C9H13NO2/c1-11-8-4-2-3-5-9(8)12-7-6-10/h2-5H,6-7,10H2,1H3
InChIKey: CKJRKLKVCHMWLV-UHFFFAOYSA-N
2-(2-Methoxyphenoxy)ethylamine Safety Profile
Hazard Codes:  Xi
Xi
RIDADR: UN2735
HazardClass: 8
PackingGroup: III
2-(2-Methoxyphenoxy)ethylamine Specification
Ethanamine,2-(2-methoxyphenoxy)- , its cas register number 1836-62-0. It also can be called 2-(2-Methoxyphenoxy)ethanamin ; and 2-(2-Methoxyphenoxy)ethanamine .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View