-
Name
4'-PHENYL-2,2':6',2''-TERPYRIDINE
- EINECS
- CAS No. 58345-97-4
- Article Data49
- CAS DataBase
- Density 1.167 g/cm3
- Solubility
- Melting Point 208 °C
- Formula C21H15N3
- Boiling Point 475.2 °C at 760 mmHg
- Molecular Weight 309.37
- Flash Point 209.4 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2,6-Bis(2-pyridyl)-4-phenylpyridine;4'-Phenyl-2,2':6',2''-terpyridine;Terosine;
- PSA 38.67000
- LogP 4.87260
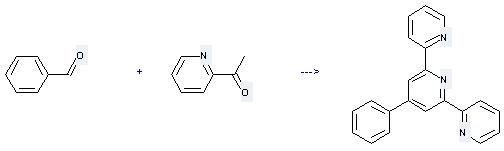
Synthetic route
-
-
96-09-3
styrene oxide

-
-
1758-54-9, 79462-42-3, 81563-77-1
1-pyridin-2-yl-ethanone oxime

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| at 200℃; for 3h; Sealed tube; | 93% |
-
-
1758-54-9, 79462-42-3, 81563-77-1
1-pyridin-2-yl-ethanone oxime

-
-
100-52-7
benzaldehyde

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 200℃; for 3h; Sealed tube; Neutral conditions; | 93% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; bis(trifluoromethanesulfonyl)amide In neat (no solvent) at 80℃; for 0.75h; | 90% |
| With [((CH3)3C)2Sn(OH)(H2O)]2(2+)*2(OSO2CF3)(1-)=[((CH3)3C)2Sn(OH)(OSO2CF3)(H2O)]2; ammonium acetate In water at 100℃; for 0.75h; Green chemistry; | 90% |
| With ammonium acetate In water at 130℃; for 0.4h; Kroehnke reaction; microwave irradiation; | 82% |

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate; oxygen at 120℃; under 760.051 Torr; for 12h; | 81% |
| With tris(pentafluorophenyl)borate; oxygen In neat (no solvent) at 120℃; under 760.051 Torr; for 12h; | 56% |
-
-
5325-66-6, 20890-12-4, 72491-18-0
1-phenyl-3-(2-pyridyl)propen-1-one

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-3-(2-pyridyl)propen-1-one; C10H10FNO3 With caesium carbonate In acetonitrile at 40℃; for 1h; Stage #2: With ammonium acetate In acetonitrile at 120℃; for 24h; regioselective reaction; | 68% |
-
-
1122-54-9
methyl (4-pyridyl) ketone

-
-
100-52-7
benzaldehyde

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Stage #1: benzaldehyde With potassium hydroxide In ethanol at 20℃; for 0.166667h; Stage #2: methyl (4-pyridyl) ketone With ammonium hydroxide In water at 20℃; for 4h; | 60% |
| With ammonium hydroxide; sodium hydroxide In ethanol at 20℃; for 12h; | 43% |
-
-
134653-69-3
4'-{[(Trifluoromethyl)sulfonyl]oxy}-2,2':6',2''-terpyridine

-
-
98-80-6
phenylboronic acid

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In tetrahydrofuran Suzuki cross-coupling reaction; Heating; | 10% |
-
-
133762-11-5
3-phenyl-1,5-bis(pyridine-2-yl)pentane-1,5-dione

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; hydroxylamine at 160℃; | |
| With ammonium acetate In acetic acid for 2h; Heating; | 4.05 g |
| With ammonium hydroxide In various solvent(s) at 100℃; for 2h; |
-
-
26482-00-8
1-(2-oxo-2-(2-pyridyl)ethyl)pyridinium iodide

-
-
53940-12-8
(E)-3-phenyl-1-(pyridin-2-yl)-2-propen-1-one

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol |
-
-
121-43-7
Trimethyl borate

-
-
89972-76-9
4'-(4-bromo-phenyl)-[2,2';6',2'']terpyridine

-
B
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 4'-(4-bromo-phenyl)-[2,2';6',2'']terpyridine With n-butyllithium at -78 - 20℃; Stage #2: Trimethyl borate at -78 - 20℃; |

-
-
61676-62-8
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
89972-76-9
4'-(4-bromo-phenyl)-[2,2';6',2'']terpyridine

-
A
-
381218-99-1
4′-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-2,2′,6′,2″-terpyridine

-
B
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 4'-(4-bromo-phenyl)-[2,2';6',2'']terpyridine With n-butyllithium at -78 - 20℃; Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane at -78 - 20℃; |

-
-
1122-62-9
2-acetylpyridine

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / various solvent(s) / 2 h / 0 °C 2: aq. NH3 / various solvent(s) / 2 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: aq. NH3; KOH / ethanol / Heating 2.1: C4H9Li / -78 - 20 °C 2.2: -78 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: aq. NH3; KOH / ethanol / Heating 2.1: C4H9Li / -78 - 20 °C 2.2: -78 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: NaOH / 0.17 h 2: 4.05 g / NH4OAc / acetic acid / 2 h / Heating View Scheme |
-
-
100-52-7
benzaldehyde

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / various solvent(s) / 2 h / 0 °C 2: aq. NH3 / various solvent(s) / 2 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: aq. NH3; KOH / ethanol / Heating 2.1: C4H9Li / -78 - 20 °C 2.2: -78 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: aq. NH3; KOH / ethanol / Heating 2.1: C4H9Li / -78 - 20 °C 2.2: -78 - 20 °C View Scheme |
-
-
100-52-7
benzaldehyde

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / 0.17 h 2: 4.05 g / NH4OAc / acetic acid / 2 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: aq. KOH / methanol 2: ammonium acetate / methanol View Scheme |
-
-
133762-11-5
3-phenyl-1,5-bis(pyridine-2-yl)pentane-1,5-dione

-
-
631-61-8
ammonium acetate

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In ethanol for 37h; Reflux; | 9.3 g |
-
-
100-42-5
styrene

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tert.-butylhydroperoxide / acetonitrile / 12 h / 80 °C 2: sodium hydroxide / ethanol View Scheme |
-
-
1122-62-9
2-acetylpyridine

-
-
108295-45-0
4′-formyl-2,2′:6′2″-terpyridine

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| With ammonium acetate; sodium hydroxide In ethanol Inert atmosphere; Schlenk technique; |
-
-
14099-01-5
rhenium(I) pentacarbonyl chloride

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
515125-29-8
[Re(CO)3(4'-phenyl-2,2':6',2''-terpyridine)Cl]

| Conditions | Yield |
|---|---|
| In toluene the mixt. in toluene was refluxed under N2 for 4 h, cooled; ppt. was filtered, stirred in DCM for 10 min, filtered, washed with CH2Cl2 and diethyl ether, dried in vac.; | 99% |
| In acetonitrile Reflux; | 75% |
| In methanol; toluene for 3h; Reflux; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| With pyridine at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine at 150℃; for 0.166667h; Inert atmosphere; Sealed tube; | 99% |
-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
133598-07-9
{Cl2(4'-phenyl-2,2':6',2''-terpyridine)manganese(II)}

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | 98% |
| In methanol refluxing for 30 min; concn., washing of the ppt. with MeOH and Et2O and drying (vac.); elem. anal.; |
-
-
110-86-1
pyridine

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| at 80℃; for 8h; Inert atmosphere; Sealed tube; | 98% |


-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
133598-07-9
{Cl2(4'-phenyl-2,2':6',2''-terpyridine)manganese(II)}

| Conditions | Yield |
|---|---|
| In methanol; acetone ligand dissolved in acetone at 50°C, a soln. of Mn salt in MeOH added, incubated at 50°C; cooled to 0°C, filtered, washed (MeOH/acetone, Et2O), dried (vac., overnight); elem. anal.; | 96% |
| In ethanol; chloroform at 20℃; Reflux; | 77% |
-
-
1271489-38-3, 32648-22-9
RuCl3(isopropyl-S-phenyl)2(CH3OH)

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
146164-70-7
{Ru(4'-Ph-2,2':6',2''-terpyridine)Cl3}

| Conditions | Yield |
|---|---|
| In acetonitrile for 18h; Reflux; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine at 250℃; for 34h; Inert atmosphere; Sealed tube; | 95% |

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile at 80℃; for 48h; High pressure; Autoclave; | 94.8% |
-
-
31355-55-2, 39210-30-5
trichlorotris(tetrahydrofuran)molybdenum(III)

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| With pyridine at 250℃; for 34h; Inert atmosphere; Sealed tube; | 94% |
-
-
14099-01-5
rhenium(I) pentacarbonyl chloride

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In methanol; toluene for 3h; Reflux; Inert atmosphere; | 93% |

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
194653-41-3
[Pt(4'-phenyl-2,2':6',2''-terpyridine)(Me)]Cl

| Conditions | Yield |
|---|---|
| In methanol byproducts: DMSO; stirring equimolar amts. for 20 min; concn. (vac.), pptn. on Et2O addn. and cooling, collection, washing (Et2O), drying (vac.); elem. anal.; | 92% |
-
-
12080-32-9
dichloro( 1,5-cyclooctadiene)platinum(ll)

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: dichloro( 1,5-cyclooctadiene)platinum(ll); silver trifluoromethanesulfonate In dichloromethane; acetonitrile for 0.5h; Darkness; Stage #2: 4'-phenyl-2,2':6',2-terpyridine In dichloromethane; acetonitrile at 20℃; for 72h; Darkness; | 92% |

-
-
14220-21-4
rhenium(I) pentacarbonyl bromide

-
-
58345-97-4
4'-phenyl-2,2':6',2-terpyridine

| Conditions | Yield |
|---|---|
| In toluene for 4h; Reflux; | 91% |
2,2':6',2''-Terpyridine,4'-phenyl- Specification
The 2,2':6',2''-Terpyridine,4'-phenyl-, also known as 4'-Phenyl-2,2':6',2''-terpyridin, is the organic compound with the formula C21H15N3. With the CAS registry number 58345-97-4, its systematic name is called 4'-phenyl-2,2':6',2''-terpyridine.
Physical properties of 2,2':6',2''-Terpyridine,4'-phenyl-: (1)ACD/LogP: 4.57; (2)ACD/LogD (pH 5.5): 4.53; (3)ACD/LogD (pH 7.4): 4.56; (4)ACD/BCF (pH 5.5): 1584.83; (5)ACD/BCF (pH 7.4): 1732.51; (6)ACD/KOC (pH 5.5): 6622.22; (7)ACD/KOC (pH 7.4): 7239.27; (8)#H bond acceptors: 3; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.63; (11)Molar Refractivity: 94.3 cm3; (12)Molar Volume: 264.9 cm3; (13)Surface Tension: 51.5 dyne/cm; (14)Density: 1.167 g/cm3; (15)Flash Point: 209.4 °C; (16)Enthalpy of Vaporization: 71.04 kJ/mol; (17)Boiling Point: 475.2 °C at 760 mmHg; (18)Vapour Pressure: 9.73E-09 mmHg at 25°C.
Preparation: this chemical can be prepared by 1-pyridin-2-yl-ethanone and benzaldehyde under other conditions of microwave irradiation and atmospheric pressure. This reaction will need reagent NH4OAc and solvent ethane-1,2-diol. The yield is about 80%.
Uses of 2,2':6',2''-Terpyridine,4'-phenyl-: it can be used to prepare 4'-phenyl-[2,2';6',2'']terpyridine 1,1''-dioxide. This reaction will need reagent 3-chloroperbenzoic acid and solvent CH2Cl2. The yield is about 60%.
![2,2':6',2''-Terpyridine,4'-phenyl- can be used to prepare 4'-phenyl-[2,2';6',2'']terpyridine 1,1''-dioxide](/UserFilesUpload/Uses of 2,2'6',2''-Terpyridine,4'-phenyl-.png)
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc(cc1)c2cc(nc(c2)c3ccccn3)c4ccccn4
(2)InChI: InChI=1/C21H15N3/c1-2-8-16(9-3-1)17-14-20(18-10-4-6-12-22-18)24-21(15-17)19-11-5-7-13-23-19/h1-15H
(3)InChIKey: RTECKDZOLWRAGK-UHFFFAOYAH
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 58348-14-4
- 583-48-2
- 58349-17-0
- 58349-23-8
- 583-50-6
- 58351-48-7
- 5835-26-7
- 583-52-8
- 58353-15-4
- 58353-68-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View