-
Name
2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide
- EINECS 209-339-0
- CAS No. 572-09-8
- Article Data343
- CAS DataBase
- Density 1.49 g/cm3
- Solubility decomposes in water
- Melting Point 86-89 °C
- Formula C14H19BrO9
- Boiling Point 412 °C at 760 mmHg
- Molecular Weight 411.203
- Flash Point 203 °C
- Transport Information
- Appearance white fluffy solid
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms a-D-Glucopyranosyl bromide,2,3,4,6-tetraacetate;Glucopyranosylbromide, tetraacetate, a-D- (8CI);1-Bromo-2,3,4,6-tetra(O-acetyl)-a-D-pyranoglucose;2,3,4,6-Tetra-O-acetyl-1-a-bromo-D-glucopyranose;2,3,4,6-Tetraacetyl-a-D-glucopyranosyl bromide;Acetobromo-D-glucose;Acetobromoglucose;D-Acetobromoglucose;Tetra-O-acetyl-a-D-glucopyranosylbromide;Tetra-O-acetyl-a-D-glucosyl bromide;a-Acetobromoglucose;a-D-Acetobromoglucose;
- PSA 114.43000
- LogP 0.46440
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen bromide In dichloromethane Ambient temperature; | 100% |
| With hydrogen bromide; acetic acid at 20℃; for 4h; | 100% |
| With hydrogen bromide at 20℃; for 1.16667h; | 100% |
-
-
83-87-4, 604-68-2, 604-69-3, 2152-77-4, 4026-35-1, 4163-59-1, 4163-60-4, 4163-61-5, 4163-65-9, 4257-94-7, 4257-96-9, 16299-15-3, 19186-39-1, 19189-55-0, 25878-60-8, 25941-03-1, 32445-48-0, 32445-49-1, 32445-53-7, 32445-54-8, 34685-58-0, 34685-59-1, 43168-42-9, 43168-44-1, 43169-22-8, 43169-25-1, 66966-07-2, 70749-17-6, 80184-01-6, 93221-01-3, 93221-02-4, 99630-88-3, 109215-53-4, 115792-70-6, 115792-73-9, 139894-92-1, 139973-25-4, 144071-49-8, 147648-81-5
D-glucose pentaacetate

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 20℃; Product distribution / selectivity; | 100% |
| With hydrogen bromide; acetic acid at 20℃; for 2h; | 100% |
| With hydrogen bromide; acetic acid In dichloromethane at 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid | 100% |
| Stage #1: α-D-glucopyranose peracetylate With hydrogen bromide; acetic acid Stage #2: at 0 - 20℃; for 2h; | 98% |
| With acetic anhydride; phosphorus tribromide In water at 0℃; for 1h; | 95% |
-
-
492-62-6
alpha-D-glucopyranose

-
-
506-96-7
Acetyl bromide

-
-
108-24-7
acetic anhydride

-
-
64-19-7
acetic acid

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With perchloric acid In methanol at 20℃; for 2h; | 99% |

-
-
2872-65-3, 14581-81-8, 17042-40-9, 84380-06-3, 105260-62-6
p-methoxyphenyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With Acetyl bromide; zinc dibromide In dichloromethane at 22℃; for 24h; | 97% |
-
-
3947-62-4, 6207-76-7, 19235-21-3, 22554-70-7, 22860-22-6, 47339-09-3, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 10343-06-3
2,3,4,5-tetra-O-acetyl-D-glucopyranose

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In dichloromethane at 0℃; for 8h; | 96% |
| With pyridine; (PhO)3P*Br2 In dichloromethane for 0.0833333h; | 89% |
| With hydrogen bromide; acetic anhydride In dichloromethane at 20℃; for 4h; | 85% |
-
-
2280-44-6
D-Glucose

-
-
108-24-7
acetic anhydride

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: D-Glucose With Acetyl bromide In methanol Stage #2: acetic anhydride With acetic acid | 95% |
| Stage #1: D-Glucose; acetic anhydride With perchloric acid; acetic acid at 20℃; for 0.5h; Stage #2: With Acetyl bromide In methanol for 2h; | 90% |
| Stage #1: D-Glucose; acetic anhydride With sodium acetate at 100℃; for 5h; Stage #2: With hydrogen bromide; acetic acid In dichloromethane at 20℃; for 6h; | 90% |
-
-
492-62-6
alpha-D-glucopyranose

-
-
108-24-7
acetic anhydride

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: alpha-D-glucopyranose; acetic anhydride With perchloric acid at 30 - 40℃; for 1h; Stage #2: With phosphorus; bromine at 20℃; Cooling with ice; Stage #3: With water at 20℃; for 3.16667h; | 90% |
| With hydrogen bromide; acetic acid at 20℃; for 11h; | 89% |
| With hydrogen bromide; acetic acid at 20℃; for 11h; | 85.88% |
-
-
3891-59-6
Penta-O-acetyl-aldehydo-D-glucose

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In dichloromethane; acetic acid at 20℃; for 6h; | 86% |
| With hydrogen bromide In acetic acid |
-
-
160227-12-3
4-methoxyphenyl 2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-β-D-glucopyranoside

-
A
-
4753-07-5
2,3,6,2',3',4',6'-hepta-O-acetyl-lactosyl bromide

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
C
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With Acetyl bromide; zinc dibromide In dichloromethane at 22℃; Yields of byproduct given; | A 82% B n/a C n/a |

-
-
231607-35-5
ethyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl disulfide

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; iodine(I) bromide In dichloromethane at 20℃; | 82% |
| With iodine(I) bromide In dichloromethane at 20℃; for 0.5h; | 82% |
-
-
100432-86-8
1-deoxy-1-[(R/S)-(phenyl)sulfinyl]-2,3,4,6-tetra-O-acetyl-β-D-glucopyranose

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane Irradiation; | 78% |
| With N-Bromosuccinimide In tetrachloromethane Product distribution; Irradiation; other glucopyranoside, other reagent, other solvent; |

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; | 78% |

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In dichloromethane; toluene at 20℃; for 1h; Molecular sieve; Inert atmosphere; | 78% |
-
-
3947-62-4, 6207-76-7, 19235-21-3, 22554-70-7, 22860-22-6, 47339-09-3, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 10343-06-3
2,3,4,5-tetra-O-acetyl-D-glucopyranose

-
-
73630-93-0
1-bromo-N,N,2-trimethyl-1-propen-1-amine

-
A
-
21678-37-5
N,N,2-trimethylpropionamide

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| In chloroform for 6h; Product distribution; Ambient temperature; | A n/a B 77% |

-
-
604-70-6
Acetic acid (2R,3R,4S,5R,6S)-3,5-diacetoxy-2-acetoxymethyl-6-methoxy-tetrahydro-pyran-4-yl ester

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With acyl bromide; zinc dibromide In dichloromethane for 12h; Heating; | 75% |
| With trimethylsilyl bromide; zinc dibromide In dichloromethane for 24h; Ambient temperature; | 47% |
-
-
4860-85-9
methyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With acyl bromide; zinc dibromide In dichloromethane for 12h; Heating; | 71% |
-
-
100432-86-8
1-deoxy-1-[(R/S)-(phenyl)sulfinyl]-2,3,4,6-tetra-O-acetyl-β-D-glucopyranose

-
A
-
3947-62-4, 6207-76-7, 19235-21-3, 22554-70-7, 22860-22-6, 47339-09-3, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 10343-06-3
2,3,4,5-tetra-O-acetyl-D-glucopyranose

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With Bromotrichloromethane In tetrachloromethane Irradiation; | A 30% B 65% |
-
-
2280-44-6
D-Glucose

-
-
506-96-7
Acetyl bromide

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: D-Glucose With perchloric acid; acetic anhydride at 30 - 40℃; for 0.5h; Stage #2: Acetyl bromide With water at 20℃; for 2h; | 57.5% |
| With aluminum oxide In acetonitrile at 20℃; for 4h; | 20% |
| In acetic acid for 2h; Ambient temperature; | |
| With sulfuric acid |
-
-
6378-65-0
n-hexyl caproate

-
-
604-69-3
β-D-glucose pentaacetate

-
A
-
142-62-1
hexanoic acid

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With bromine at 20℃; Irradiation; Green chemistry; | A n/a B 57% |

-
-
112-17-4
n-decyl acetate

-
-
604-69-3
β-D-glucose pentaacetate

-
A
-
112-29-8
1-bromo dodecane

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With bromine at 20℃; Irradiation; Green chemistry; | A n/a B 57% |


-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 20℃; for 2h; | 53% |
-
-
604-69-3
β-D-glucose pentaacetate

-
A
-
29585-29-3
1,3,4,6-tetra-O-acetyl 2-bromo-2-deoxy-α-D-glucopyranose

-
B
-
3947-62-4, 6207-76-7, 19235-21-3, 22554-70-7, 22860-22-6, 47339-09-3, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 10343-06-3
2,3,4,5-tetra-O-acetyl-D-glucopyranose

-
C
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With bromine In 2,2,4-trimethylpentane; dichloromethane at 20℃; for 7h; Irradiation; Green chemistry; | A 7% B 15% C 22% |

-
-
1138026-28-4
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl-1-C-sulfonamide

-
C
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With bromine; potassium carbonate In α,α,α-trifluorotoluene for 4h; Reflux; Heat lamp; | A 12% B 12% C 19% |

-
-
604-69-3
β-D-glucose pentaacetate

-
A
-
69534-61-8
1,2,3,4,6-Penta-O-acetyl-5-bromo-β-D-glucopyranose

-
B
-
75868-36-9
2,3,4,6-tetra-O-acetyl-1-bromo-D-glucopyranosyl bromide

-
C
-
75860-51-4
2,3,4,6-tetra-O-acetyl-5-bromo-β-D-glucopyranosyl bromide

-
D
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 3h; Heating; Irradiation; | A n/a B n/a C 13% D 0.06 g |
| With bromine In tetrachloromethane for 3h; Heating; Irradiation; Yield given; | A n/a B n/a C 13% D 0.06 g |
| With bromine In tetrachloromethane for 3h; Heating; Irradiation; Yields of byproduct given; | A n/a B n/a C 13% D 0.06 g |

-
-
604-69-3
β-D-glucose pentaacetate

-
A
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
B
-
604-68-2
α-D-glucopyranose peracetylate

| Conditions | Yield |
|---|---|
| With bromine In ethyl acetate at 20℃; for 19h; Irradiation; Darkness; Green chemistry; | A 8% B n/a |

| Conditions | Yield |
|---|---|
| With Acetyl bromide; acetic acid | |
| Multi-step reaction with 2 steps 1.1: pyridine / 1 h / 0 - 20 °C / Inert atmosphere 1.2: 6 h / 20 °C / Inert atmosphere 2.1: hydrogen bromide; acetic acid / dichloromethane / 1 h / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / 6 h / 0 °C 2: acetic acid; hydrogen bromide / dichloromethane / 2 h View Scheme |
-
-
50-99-7
D-glucose

-
-
108-24-7
acetic anhydride

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With perchloric acid anschl. mit Phosphor, Brom und H2O; | |
| With pyridine; hydrogen bromide; acetic acid Yield given; Multistep reaction; | |
| Stage #1: D-glucose; acetic anhydride With iodine at 20℃; for 1h; Stage #2: With hydrogen bromide; acetic acid | |
| Stage #1: D-glucose; acetic anhydride With perchloric acid In water at 40℃; for 1h; Stage #2: With phosphorus; bromine In water at 0 - 20℃; for 4h; |
-
-
77938-63-7
sodium chloride * 2 d-glucose * H2O

-
-
108-24-7
acetic anhydride

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With sulfuric acid anschl. Behandeln des von der gebildeten Essigsaeure befreiten Rktprod. mit HBr; |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
13992-25-1
1-azido-1-deoxy-β-D-glucopyranoside tetraacetate

| Conditions | Yield |
|---|---|
| With tetramethylguanidinum azide In nitromethane at 25℃; for 2.5h; | 100% |
| With sodium azide In N,N-dimethyl-formamide for 48h; Inert atmosphere; | 100% |
| With N,N,N',N'-tetramethylguanidinium azide In dichloromethane for 2h; Ambient temperature; other glycosyl halides; | 99% |
-
-
592-87-0
lead(II) thiocyanate

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
14152-97-7
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate

| Conditions | Yield |
|---|---|
| In benzene for 6h; Heating; | 100% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 130 - 140℃; for 0.416667h; Microwave irradiation; | 89.66% |
| In toluene for 4h; Reflux; | 89% |
-
-
17356-08-0
thiourea

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
40591-65-9
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiouronium bromide

| Conditions | Yield |
|---|---|
| In acetone at 60℃; Inert atmosphere; Reflux; | 100% |
| In acetone for 0.5h; Heating; | 95% |
| In acetone for 0.05h; microwave irradiation; | 90% |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
165053-19-0
3,4,6-tri-O-acetyl-1,2-O-vinylidene-α-D-glucopyranose

| Conditions | Yield |
|---|---|
| With silver perchlorate; N-ethyl-N,N-diisopropylamine In benzene at 20℃; for 1h; Molecular sieve; Inert atmosphere; Darkness; | 100% |
| With N-ethyl-N,N-diisopropylamine; silver(l) oxide In benzene for 6h; Heating; |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
724733-04-4
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-undecanoic acid (4-mercapto-phenyl)-methyl-amide

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; ethyl acetate at 20℃; for 2h; | 100% |

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide; phase transfer catalyst In dichloromethane at 20℃; for 0.5h; | 100% |
-
-
612-25-9
2-Nitrobenzyl alcohol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
34546-55-9
2-nitrobenzyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With iodine; silver carbonate In dichloromethane at 20℃; for 16h; Molecular sieve; Inert atmosphere; | 100% |
| With iodine; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile for 4h; Molecular sieve; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 80℃; | 100% |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
3947-62-4, 6207-76-7, 19235-21-3, 22554-70-7, 22860-22-6, 47339-09-3, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 10343-06-3
2,3,4,5-tetra-O-acetyl-D-glucopyranose

| Conditions | Yield |
|---|---|
| With water; silver carbonate In acetone for 0.5h; | 99% |
| With N-methylacridinium iodide; sodium cyanoborohydride In N,N-dimethyl-formamide for 1h; Reagent/catalyst; Solvent; UV-irradiation; | 55.8% |
| With acetone; silver carbonate |
-
-
77-52-1
ursolic acid

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
16684-20-1
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl 3β-hydroxyurs-12-en-28-oate

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In dichloromethane; water for 8h; Heating; | 99% |
| With Aliquat 336; potassium carbonate In dichloromethane; water for 48h; Ambient temperature; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 90% |
-
-
65-85-0
benzoic acid

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
38430-69-2
O2,O3,O4,O6-Tetraacetyl-O1-benzoyl-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Reagent/catalyst; Molecular sieve; | 99% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Molecular sieve; Inert atmosphere; | 84% |
| With Amberlyst A-26 hydroxide form 1.) hexane, 2.) benzene, reflux, 12 h; | 65% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Molecular sieve; | 99% |
| With Aliquat 336; potassium carbonate In dichloromethane; water Ambient temperature; | 96% |
-
-
148-18-5
sodium N,N-diethyldithiocarbamate

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| In acetone for 1h; Reflux; | 99% |
| In acetonitrile at 25℃; for 1h; | 63% |
-
-
149-30-4
2-thioxo-3H-1,3-benzothiazole

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
6067-32-9, 6426-38-6
2-(2',3',4',6'-tetra-O-acetyl-β-D-glucopyranosylmercapto)1,3-benzothiazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetonitrile at 20℃; for 1h; | 99% |
-
-
1095998-03-0
4-(pyridin-2'-yl)thiazole-2(3H)-thione sodium salt

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
1095998-22-3
4-(pyridine-2-yl)-thiazole-2-yl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 8h; Inert atmosphere; | 99% |
-
-
74247-81-7
6-O-tert-butyldimethylsilanyl-1-O-methyl-α-D-mannopyranoside

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
1330004-16-4
methyl 6-O-tert-butyldimethylsilyl-3-O-(2′,3′,4′,6′-tetra-O-acetyl-β-D-glucopyranosyl)-α-D-mannopyranoside

| Conditions | Yield |
|---|---|
| With diphenylborate ethanolamine ester; silver(l) oxide In acetonitrile at 23℃; for 16h; Kinetics; Mechanism; Concentration; Reagent/catalyst; Koenigs-Knorr synthesis; Inert atmosphere; regioselective reaction; | 99% |
| With [2-(1-methyl-1H-imidazol-2-yl)phenyl]boronic acid; silver(l) oxide In acetonitrile at 30℃; for 24h; Reagent/catalyst; Koenigs-Knorr Glycosidation; Inert atmosphere; regioselective reaction; | 98% |
-
-
181480-80-8
6-O-tert-butyldimethylsilanyl-1-O-methyl-α-D-galactopyranoside

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
1330004-23-3
methyl 6-O-tert-butyldimethylsilyl-3-O-(2’,3’,4’,6’-tetra-O-acetyl-β-D-glucopyranosyl)-α-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With [2-(1-methyl-1H-imidazol-2-yl)phenyl]boronic acid; silver(l) oxide In acetonitrile at 30℃; for 24h; Koenigs-Knorr Glycosidation; Inert atmosphere; regioselective reaction; | 99% |
| With diphenylborate ethanolamine ester; silver(l) oxide In acetonitrile at 23℃; for 16h; Koenigs-Knorr synthesis; Inert atmosphere; regioselective reaction; | 74% |
| With diphenylborate ethanolamine ester; silver(l) oxide In acetonitrile at 23℃; for 16h; Reagent/catalyst; regioselective reaction; | 74% |
-
-
617-04-9
(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol With diphenyltin(IV) dichloride In acetonitrile at 20℃; for 0.166667h; Stage #2: 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide With 5,5'-dimethyl-2,2'-bipyridine; silver(l) oxide In acetonitrile at 20 - 35℃; for 24h; Reagent/catalyst; Koenigs-Knorr Glycosidation; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Molecular sieve; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Molecular sieve; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Molecular sieve; | 99% |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
10387-40-3
potassium thioacetate

-
-
6806-56-0, 13639-50-4, 51898-27-2, 62860-10-0, 63783-77-7, 92217-67-9, 92217-68-0, 130796-15-5
O2,O3,O4,O6,S-Pentaacetyl-1-thio-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| for 2h; Neat (no solvent); Ball milling; | 98% |
| In acetone at 20℃; for 8h; | 88% |
| In acetone at 20℃; | 86% |
-
-
107-18-6
allyl alcohol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
10343-15-4, 39698-55-0, 54400-76-9, 78730-32-2, 119111-31-8, 119111-32-9
Acetic acid (2R,3R,4S,5R,6R)-3,5-diacetoxy-2-acetoxymethyl-6-allyloxy-tetrahydro-pyran-4-yl ester

| Conditions | Yield |
|---|---|
| With copper(II) sulfate; mercury dibromide; mercury(II) oxide In chloroform at 0 - 20℃; for 10h; | 98% |
| With indium(III) chloride; 4 A molecular sieve In dichloromethane at 0 - 3℃; for 72h; | 86% |
| With metal carbonates In neat (no solvent) for 2.5h; Milling; | 82% |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
13137-69-4
1-deoxy-D-glucose tetraacetate

| Conditions | Yield |
|---|---|
| With -butyl vinyl ether | 98% |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene at 80℃; for 1h; | 97% |
| With 2,2'-azobis(isobutyronitrile); phenylsilane for 1.16667h; Heating; | 90% |
-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
439-03-2, 439-04-3, 439-05-4, 2823-44-1, 2823-46-3, 3934-29-0, 4163-44-4, 4163-45-5, 20409-31-8, 23235-96-3, 51897-75-7, 51897-76-8, 51897-77-9, 57573-38-3, 62561-28-8, 126641-47-2
2-(acetoxymethyl)-6-fluorotetrahydro-2H-pyran-3,4,5-triyl triacetate

| Conditions | Yield |
|---|---|
| With silver fluoride In acetonitrile at 20℃; Inert atmosphere; Darkness; | 98% |
| With triethylamine tris(hydrogen fluoride) In tetrachloromethane for 2h; Heating; | 83% |
| With (trifluoromethyl)zinc chloride; 3 A molecular sieve In dichloromethane for 12h; Ambient temperature; | 83% |
| With potassium hydrogen bifluoride In acetonitrile for 24h; Heating; | 70% |
| With silver fluoride; acetonitrile |
-
-
4064-06-6
1,2:3,4-di-O-isopropylidene-α-D-galactopyranose

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
66964-24-7
3,4,6-Tri-O-acetyl-1,2-O-<1-(exo-1,2:3,4-di-O-isopropylidene-α-D-galacatopyranose-6-yl)ethylidene>-α-D-glucopyranose

| Conditions | Yield |
|---|---|
| With silver imidazolate; mercury dichloride In dichloromethane for 24h; 3 Angstroem molecular sieves; | 98% |
| With 4 A molecular sieve; tetrabutylammomium bromide; N-ethyl-N,N-diisopropylamine In dichloromethane at 40℃; under 6000480 Torr; for 20h; Yield given; |
2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide Chemical Properties
Product Name: Acetobromglucose (CAS NO.572-09-8)
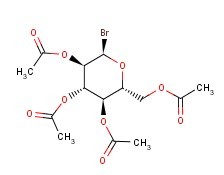
Molecular Formula: C14H19BrO9
Molecular Weight: 411.20g/mol
Mol File: 572-09-8.mol
EINECS: 209-339-0
Appearance: White powder
Melting Point: 83-88 ºC
Boiling point: 412 °C at 760 mmHg
Flash Point: 203 °C
Density: 1.49 g/cm3
Water Solubility: Decomposes
Surface Tension: 47.9 dyne/cm
Enthalpy of Vaporization: 66.46 kJ/mol
Vapour Pressure: 5.35E-07 mmHg at 25°C
XLogP3-AA: 1.5
H-Bond Donor: 0
H-Bond Acceptor: 9
Structure Descriptors of Acetobromglucose (CAS NO.572-09-8):
IUPAC Name: [(2R,3R,4S,5R,6R)-4,5-diacetyloxy-2-(acetyloxymethyl)-6-bromooxan-3-yl]acetate
Canonical SMILES: CC(=O)OCC1C(C(C(C(O1)Br)OC(=O)C)OC(=O)C)OC(=O)C
Isomeric SMILES: CC(=O)OC[C@@H]1[C@H]([C@@H]([C@H]([C@H](O1)Br)OC(=O)C)OC(=O)C)OC(=O)C
InChI: InChI=1S/C14H19BrO9/c1-6(16)20-5-10-11(21-7(2)17)12(22-8(3)18)13(14(15)24-10)23-9(4)19/h10-14H,5H2,1-4H3/t10-,11-,12+,13-,14+/m1/s1
InChIKey: CYAYKKUWALRRPA-RGDJUOJXSA-N
2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide Safety Profile
Safety Information of Acetobromglucose (CAS NO.572-09-8):
Safety Statements: S24/25
24: Avoid contact with skin
25: Avoid contact with eyes
2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide Specification
Acetobromglucose , its CAS NO. is 572-09-8, the synonyms are EINECS 209-339-0 ; 2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide ; alpha-D-Glucopyranosyl bromide, tetraacetate .
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 5721-12-0
- 57212-34-7
- 57212-78-9
- 57213-48-6
- 57213-69-1
- 5721-37-9
- 57214-69-4
- 57215-97-1
- 5721-91-5
- 57219-64-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View