-
Name
2,4,6-Trihydroxybenzoic acid
- EINECS 201-467-5
- CAS No. 83-30-7
- Article Data29
- CAS DataBase
- Density 1.749 g/cm3
- Solubility
- Melting Point ~210 °C (dec.)
- Formula C7H6O5
- Boiling Point 426.5 °C at 760 mmHg
- Molecular Weight 170.122
- Flash Point 225.9 °C
- Transport Information
- Appearance Light beige crystalline powder.
- Safety 22-24/25-26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,4,6-Trihydroxybenzenecarboxylicacid;NSC 36720;Phloroglucinic acid;Phloroglucinolcarboxylic acid;
- PSA 97.99000
- LogP 0.50160
2,4,6-Trihydroxybenzoic acid Consensus Reports
Reported in EPA TSCA Inventory.
2,4,6-Trihydroxybenzoic acid Specification
The IUPAC name of this chemical is 2,4,6-Trihydroxybenzoic acid. With the CAS registry number 83-30-7 and EINECS registry number 201-467-5, it is also named as benzoic acid, 2,4,6-trihydroxy-. In addition, the molecular formula is C7H6O5. It is a trihydroxybenzoic acid. Besides, it is a catechin degradation product excreted by the bacterium Acinetobacter calcoaceticus. And it should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 1.80; (2)ACD/LogD (pH 5.5): -1.35; (3)ACD/LogD (pH 7.4): -1.37; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 5; (9)#H bond donors: 4; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 53.99 Å2; (12)Index of Refraction: 1.73; (13)Molar Refractivity: 38.82 cm3; (14)Molar Volume: 97.2 cm3; (15)Polarizability: 15.39 ×10-24cm3; (16)Surface Tension: 109.2 dyne/cm; (17)Density: 1.749 g/cm3; (18)Flash Point: 225.9 °C; (19)Enthalpy of Vaporization: 71.82 kJ/mol; (20)Boiling Point: 426.5 °C at 760 mmHg; (21)Vapour Pressure: 4.93E-08 mmHg at 25°C.
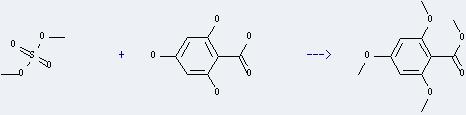
Uses of 2,4,6-Trihydroxybenzoic acid: it can react with sulfuric acid dimethyl ester to get 2,4,6-trimethoxy-benzoic acid methyl ester. This reaction will need reagent K2CO3 and solvent acetone. The reaction time is 26 hours at reaction temperature of 42-45 °C. The yield is about 48%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. During using it, wear gloves and eye/face protection and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, you should not breathe dust.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)c1c(O)cc(O)cc1O
(2)InChI: InChI=1/C7H6O5/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2,8-10H,(H,11,12)
(3)InChIKey: IBHWREHFNDMRPR-UHFFFAOYAP
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 800mg/kg (800mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 196, Pg. 478, 1976. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 83310-97-8
- 83-31-8
- 83320-61-0
- 83322-02-5
- 83324-51-0
- 83-32-9
- 83329-89-9
- 83-33-0
- 83335-41-5
- 83-34-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View