-
Name
2-Amino-6-fluorobenzonitrile
- EINECS
- CAS No. 77326-36-4
- Article Data10
- CAS DataBase
- Density 1.256 g/cm3
- Solubility
- Melting Point 125-128 °C(lit.)
- Formula C7H5FN2
- Boiling Point 275.775 °C at 760 mmHg
- Molecular Weight 136.129
- Flash Point 120.585 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 26-36/37/39-22
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, T,
T, Xn
Xn
- Synonyms 2-Amino-6-fluoro benzonitrile;2-Amino-6-fluorobenzonitrile(77326-36-4);2-Fluoro-6-aminobenzonitrile;2-Amino-6-fluorobenzo-nitrile;Benzonitrile, 2-amino-6-fluoro-;
- PSA 49.81000
- LogP 1.86078
Synthetic route
-
-
1897-52-5
2,6 difluorobenzonitrile

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With ammonia In dimethyl sulfoxide at 80℃; for 80h; closed bottle; | 99% |
| With ammonia In ethanol at 140℃; under 10343.2 Torr; for 6h; | 97% |
| With ammonia In dimethyl sulfoxide at 80 - 120℃; | 97.5% |

| Conditions | Yield |
|---|---|
| With boron trichloride; tin(IV) chloride; potassium carbonate 1.) CH2Cl2, reflux, 24 h, 2.) CH3OH, reflux, 2.5 h; Yield given. Multistep reaction; |
-
-
1194-65-6
2,6-dichloro-benzonitrile

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KF; 18-crown-6 / 3 h / 180 °C 2: NH3 / 10 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| In formamide |

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; oxygen In tert-Amyl alcohol at 110℃; under 2250.23 Torr; for 24h; Autoclave; Green chemistry; | 92 %Chromat. |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
115643-59-9
2-amino-6-fluorobenzamide

| Conditions | Yield |
|---|---|
| With water; potassium carbonate at 150℃; for 0.25h; Microwave irradiation; | 99% |
| With water; potassium carbonate at 150℃; for 0.5h; Microwave irradiation; | 94% |
| With water; potassium carbonate In water at 150℃; for 0.5h; Microwave irradiation; | 94% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

| Conditions | Yield |
|---|---|
| at 100℃; for 0.5h; | 99% |
| In toluene at 120℃; for 1.5h; |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
329-15-7
4-trifluoromethyl-phenyl acetyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 16h; Inert atmosphere; | 99% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
541-41-3
chloroformic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 18h; | 99% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfide monohydrate; magnesium chloride In N,N-dimethyl-formamide at 20℃; for 72h; Sealed tube; | 97% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
845866-92-4
6-amino-3-bromo-2-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; acetic acid at 30℃; for 18h; Temperature; | 95.3% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
103-72-0
phenyl isothiocyanate

-
-
1098337-03-1
4-anilino-5-fluoroquinazoline-2-thiol

| Conditions | Yield |
|---|---|
| at 100℃; for 3.5h; | 95% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
103-72-0
phenyl isothiocyanate

| Conditions | Yield |
|---|---|
| at 100℃; for 3.5h; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 160℃; for 5h; | 95% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
192570-33-5
5-fluoro-1H-quinazoline-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With carbon dioxide In water at 150℃; under 37503.8 Torr; for 5h; | 94.8% |
-
-
108-86-1
bromobenzene

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
606145-77-1
N-(2-cyano-3-fluorophenyl)benzonitrile

| Conditions | Yield |
|---|---|
| With palladium diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene; catacxium A In N,N-dimethyl-formamide at 130℃; for 16h; Inert atmosphere; Sealed tube; | 92% |

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1600526-88-2
2,2'-(triazene-1,3-diyl)-bis(6-fluorobenzonitrile)

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 0 - 20℃; for 20h; | 92% |
-
-
67-56-1
methanol

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
86505-94-4
2-amino-6-fluoro-benzoic acid,methyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride Pinner reaction; | 91% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1711-07-5
3-fluorobenzoyl chloride

-
-
1153761-87-5
N-(2-cyano-3-fluorophenyl)-3-fluorobenzamide

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 3h; | 91% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
456-48-4
3-Fluorobenzaldehyde

-
-
1153511-93-3
2-fluoro-6-(3-fluorobenzylamino)benzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-fluorobenzonitrile; 3-Fluorobenzaldehyde With trifluoroacetic acid In acetic acid at 35 - 40℃; for 1.5h; Large scale reaction; Stage #2: With sodium tris(acetoxy)borohydride In acetic acid at 40℃; for 1h; | 90.3% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
434-76-4
2-amino-6-fluorobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 12h; Heating; | 90% |
| With sodium hydroxide In ethanol | |
| With sodium hydroxide In ethanol; water |
-
-
402948-37-2
[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]acetic acid ethyl ester

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
405169-16-6
4-amino-5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-quinolin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: [6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]acetic acid ethyl ester; 2-amino-6-fluorobenzonitrile In toluene at 20 - 65℃; for 2h; Inert atmosphere; Stage #2: With potassium tert-butylate In toluene at 50℃; for 2h; Temperature; | 88.4% |
| With potassium tert-butylate In tetrahydrofuran; toluene at 17 - 30℃; for 2h; | 85.4% |
| Stage #1: [6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]acetic acid ethyl ester; 2-amino-6-fluorobenzonitrile In toluene at 63℃; Stage #2: With potassium tert-butylate In tetrahydrofuran; toluene at 17 - 30℃; for 2h; Product distribution / selectivity; | 85.4% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
83124-74-7
4-ethoxy-1,1,1-trichloro-3-buten-2-one

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 88% |
-
-
392-56-3
Hexafluorobenzene

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-fluorobenzonitrile With potassium tert-butylate In tetrahydrofuran at 60℃; Glovebox; Stage #2: Hexafluorobenzene In tetrahydrofuran at 60℃; for 5h; | 86% |
-
-
1554631-83-2
(S)-1-(3-(hydroxymethyl)piperidin-1-yl)-3-methylbutan-1-one

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1554631-80-9
(S)-2-amino-6-((1-(3-methylbutanoyl)piperidin-3-yl)methoxy)benzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: (S)-1-(3-(hydroxymethyl)piperidin-1-yl)-3-methylbutan-1-one With sodium hydride In tetrahydrofuran; mineral oil at 0 - 25℃; for 1h; Stage #2: 2-amino-6-fluorobenzonitrile In tetrahydrofuran; mineral oil Reflux; | 83.87% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
124-41-4
sodium methylate

-
-
1591-37-3
2-amino-6-methoxybenzonitrile

| Conditions | Yield |
|---|---|
| With methanol In N,N-dimethyl-formamide at 20℃; for 18h; Inert atmosphere; | 83% |
| In methanol; DMF (N,N-dimethyl-formamide) at 20℃; for 9h; Heating / reflux; | 63% |
| In methanol; N,N-dimethyl-formamide at 20℃; for 9h; Heating / reflux; | 63% |
-
-
1093206-04-2
N-(2-(benzyloxy)ethyl)-3-hydroxy-2,2-dimethylpropanamide

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1093206-03-1
3-(3-amino-2-cyanophenoxy)-N-(2-(benzyloxy)ethyl)-2,2-dimethylpropanamide

| Conditions | Yield |
|---|---|
| 82% |
-
-
201230-82-2
carbon monoxide

-
-
104-95-0
(4-bromophenyl)thioanisole

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1570097-49-2
5-fluoro-2-(4-(methylthio)phenyl)quinazolin-4(3H)-one

| Conditions | Yield |
|---|---|
| With water; palladium diacetate; potassium carbonate; catacxium A In dimethyl sulfoxide at 120℃; under 7500.75 Torr; for 16h; Inert atmosphere; Autoclave; | 82% |


-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With chloro(2-dicyclohexylphosphino-2’,4’,6’-triisopropyl-1,1‘-biphenyl)[2-(2’-amino-1,1‘-biphenyl’)]palladium(II); sodium t-butanolate In 1,4-dioxane at 90℃; for 0.75h; Buchwald-Hartwig Coupling; Microwave irradiation; | 82% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
79544-27-7
2-bromo-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-fluorobenzonitrile With sulfuric acid; sodium nitrite In water at 10℃; Stage #2: With hydrogen bromide; copper(I) bromide at 45℃; | 81% |
| Stage #1: 2-amino-6-fluorobenzonitrile With hydrogen bromide; sodium nitrite In 1,4-dioxane; water at 0℃; for 3h; Stage #2: With hydrogen bromide; copper(I) bromide In 1,4-dioxane; water at 0 - 50℃; for 0.583333h; | 70% |
| With copper(I) bromide; hydrogen bromide; sodium nitrite 1.)0 to -5 deg C, 1.5 h 2.)r.t., 15 h; Yield given. Multistep reaction; |
-
-
108-86-1
bromobenzene

-
-
201230-82-2
carbon monoxide

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1098336-86-7
5-fluor-2-phenylquinazolin-4(3Η)-one

| Conditions | Yield |
|---|---|
| With water; palladium diacetate; potassium carbonate; catacxium A In dimethyl sulfoxide at 120℃; under 7500.75 Torr; for 16h; Inert atmosphere; Autoclave; | 81% |

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
1885-29-6
anthranilic acid nitrile

-
-
1098336-86-7
5-fluor-2-phenylquinazolin-4(3Η)-one

| Conditions | Yield |
|---|---|
| Stage #1: anthranilic acid nitrile With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; butyraldehyde oxime In para-xylene at 70℃; for 12h; Inert atmosphere; Schlenk technique; Stage #2: 2-amino-6-fluorobenzonitrile In para-xylene at 110℃; for 4h; Inert atmosphere; Schlenk technique; | 80% |
-
-
77326-36-4
2-amino-6-fluorobenzonitrile

-
-
100-52-7
benzaldehyde

-
-
1098336-86-7
5-fluor-2-phenylquinazolin-4(3Η)-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-fluorobenzonitrile With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; butyraldehyde oxime In para-xylene at 70℃; for 12h; Schlenk technique; Stage #2: benzaldehyde In para-xylene at 20 - 110℃; for 4h; Schlenk technique; | 80% |
| With valeraldoxime In tert-Amyl alcohol at 125℃; for 15h; Inert atmosphere; | 80% |

-
-
77326-36-4
2-amino-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 0.352833h; Inert atmosphere; Reflux; | 80% |
2-Amino-6-fluorobenzonitrile Specification
The 2-Amino-6-fluorobenzonitrile, with the CAS registry number 77326-36-4, is also known as Benzonitrile, 2-amino-6-fluoro-. And the molecular formula of this chemical is C7H5FN2. It is a kind of white to light yellow crystal powder, and belongs to the following product categories: Nitrile; Aromatic Nitriles; Benzene series. What's more, it is used as intermediate of pesticide and medicine.
The physical properties of 2-Amino-6-fluorobenzonitrile are as following: (1)ACD/LogP: 2.17; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.165; (4)ACD/LogD (pH 7.4): 2.165; (5)ACD/BCF (pH 5.5): 26.041; (6)ACD/BCF (pH 7.4): 26.041; (7)ACD/KOC (pH 5.5): 358.87; (8)ACD/KOC (pH 7.4): 358.877; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 49.81 Å2; (13)Index of Refraction: 1.56; (14)Molar Refractivity: 35.05 cm3; (15)Molar Volume: 108.37 cm3; (16)Polarizability: 13.895×10-24cm3; (17)Surface Tension: 50.105 dyne/cm; (18)Density: 1.256 g/cm3; (19)Flash Point: 120.585 °C; (20)Enthalpy of Vaporization: 51.425 kJ/mol; (21)Boiling Point: 275.775 °C at 760 mmHg; (22)Vapour Pressure: 0.005 mmHg at 25°C.
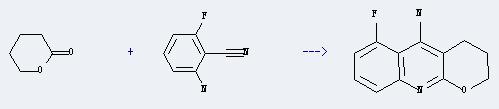
Uses of 2-Amino-6-fluorobenzonitrile: It can react with tetrahydro-pyran-2-one to produce 5-fluoro-3,4-dihydro-2H-1-oxa-9-aza-anthracen-10-ylamine. This reaction will need reagents TiCl4 and triethylamine, and the solvent CH2Cl2. The reaction time is 18 hours with ambient temperature, and the yield is about 55%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin, and it is also harmful by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Do not breathe dust; Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(c(c(c1)F)C#N)N
(2)InChI: InChI=1/C7H5FN2/c8-6-2-1-3-7(10)5(6)4-9/h1-3H,10H2
(3)InChIKey: IQUNZGOZUJITBJ-UHFFFAOYAA
Related Products
- 2-Amino-6-fluorobenzonitrile
- 77326-45-5
- 77326-62-6
- 77326-96-6
- 77327-45-8
- 77330-02-0
- 7733-02-0
- 77331-77-2
- 77332-79-7
- 77332-89-9
- 77332-90-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View