-
Name
2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride
- EINECS -0
- CAS No. 86604-75-3
- Article Data17
- CAS DataBase
- Density
- Solubility Soluble in water.
- Melting Point 128-131 °C(lit.)
- Formula C9H12ClNO.HCl
- Boiling Point 272.2 °C at 760 mmHg
- Molecular Weight 222.114
- Flash Point 118.4 °C
- Transport Information UN 3261
- Appearance White to off White crystalline powder with irritating odour
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride
- PSA 22.12000
- LogP 3.24780
Synthetic route
-
-
86604-78-6
(4-methoxy-3,5-dimethylpyridin-2-yl)methanol

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane at 20℃; for 1h; | 100% |
| With thionyl chloride In dichloromethane at 20℃; for 1h; | 100% |
| With bis(trichloromethyl) carbonate; Triphenylphosphine oxide In toluene at 20 - 60℃; for 6h; Temperature; | 98% |
-
-
84006-10-0
(chloromethyl)-4-methoxy-3,5-dimethylpyridine

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In toluene at 15℃; for 0.1h; | 94.5% |
| With hydrogenchloride In ethanol at 20℃; for 1h; Inert atmosphere; | 92.6% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 76 percent / 30percent aq. H2O2, HOAc / 23 h / 90 °C 2: HNO3 / Heating 4: 110 °C 5: aq. NaOH / methanol / Heating 6: 90 percent / SOCl2 / CH2Cl2 / 0.5 h / Heating View Scheme |
-
-
86604-79-7
2,3,5-trimethyl-4-nitropyridine N-oxide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 2: 110 °C 3: aq. NaOH / methanol / Heating 4: 90 percent / SOCl2 / CH2Cl2 / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: NaOMe / 16 h / 40 °C 2: 2 h / 90 °C 3: 2 N aq. NaOH / 2 h / 80 °C 4: SOCl2 / CH2Cl2 / 2 h / 0 - 5 °C View Scheme |
-
-
74409-42-0
2,3,5-trimethylpyridine 1-oxide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: HNO3 / Heating 3: 110 °C 4: aq. NaOH / methanol / Heating 5: 90 percent / SOCl2 / CH2Cl2 / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: 98percent HNO3 / acetic acid / 33 h / 80 °C 2: NaOMe / 16 h / 40 °C 3: 2 h / 90 °C 4: 2 N aq. NaOH / 2 h / 80 °C 5: SOCl2 / CH2Cl2 / 2 h / 0 - 5 °C View Scheme |
-
-
86604-80-0
4-methoxy-2,3,5-trimethylpyridine 1-oxide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 110 °C 2: aq. NaOH / methanol / Heating 3: 90 percent / SOCl2 / CH2Cl2 / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 2 h / 90 °C 2: 2 N aq. NaOH / 2 h / 80 °C 3: SOCl2 / CH2Cl2 / 2 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 3 steps 1: acetic acid / methanol; toluene 2: sodium hydroxide / methanol 3: thionyl chloride View Scheme | |
| Multi-step reaction with 3 steps 1: Raney nickel / methanol / 6 h / 40 °C 2: trichloroisocyanuric acid / chloroform / 4 h / 50 °C 3: hydrogenchloride / toluene / 0.1 h / 15 °C View Scheme |
-
-
91219-90-8
(4-methoxy-3,5-dimethyl-pyridin-2-yl)methyl acetate

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / methanol / Heating 2: 90 percent / SOCl2 / CH2Cl2 / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 2 N aq. NaOH / 2 h / 80 °C 2: SOCl2 / CH2Cl2 / 2 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol 2: thionyl chloride View Scheme |

-
-
86604-78-6
(4-methoxy-3,5-dimethylpyridin-2-yl)methanol


-
B
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| In chloroform |

-
-
67-63-0
isopropyl alcohol

-
-
104916-41-8
3,5-dimethyl-4-methoxy-2-cyanopyridine

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide; thionyl chloride; sodium nitrite; aluminum nickel In NH3 -saturated methanol; water; acetic acid |
-
-
96300-88-8
2-Hydroxymethyl-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane at 15 - 30℃; for 1h; | |
| With thionyl chloride |
-
-
109371-20-2
4-chloro-2,3,5-trimethylpyridine 1-oxide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydroxide 2: acetic acid / methanol; toluene 3: sodium hydroxide / methanol 4: thionyl chloride View Scheme |
-
-
651729-89-4
3-[4-(2-mercapto-5-methoxy-benzimidazole-1-sulfonyl)-phenyl]-propionic acid 2-(toluene-4-sulfonyl)ethyl ester

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
651729-90-7
3-[4-(5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)benzimidazole-1-sulfonyl)-phenyl]-propionic acid 2-(toluene-4-sulfonyl)ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 2h; | 100% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 2h; | 100% |
-
-
848694-70-2
N-{5-[2-(tert-butyl-diphenyl-silanyloxy)-ethyl]-4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl}-acetamide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
848694-71-3
N-[5-[2-(tert-butyl-diphenyl-silanyloxy)-ethyl]-4-chloro-7-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl]-acetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; | 100% |
-
-
37052-78-1
2-Mercapto-5-methoxybenzimidazole

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
73590-85-9
omeprazole sulfide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; edetate disodium; sodium hydroxide In dichloromethane; water pH=10; pH-value; | 100% |
| With sodium hydroxide at 20℃; | 98% |
| With sodium hydroxide In methanol; water Reflux; | 95.2% |
-
-
136918-14-4
phthalimide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
946071-49-4
2-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
113713-24-9
5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylthio)-1H-imidazo[4,5-b]pyridine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 25 - 30℃; Large scale reaction; | 98% |
| With sodium hydroxide In methanol; water at 25 - 30℃; for 0.75h; | 88.8% |

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With silver carbonate In toluene at 110℃; for 6h; Darkness; | 98% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
92806-76-3
5-tert-butyl-2-mercapto-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Heating; | 97% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
110-70-3
N,N`-dimethylethylenediamine

-
-
1523156-35-5
N,N′‑dimethyl‑N,N′‑bis(4‑methoxy‑3,5‑dimethylpyridine‑2‑ylmethyl)‑1,2 diaminoethane

| Conditions | Yield |
|---|---|
| Stage #1: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride With potassium carbonate In water at 20℃; for 0.5h; Stage #2: N,N`-dimethylethylenediamine With sodium hydroxide In dichloromethane; water at 20℃; for 60h; | 97% |
| With sodium hydroxide In dichloromethane; water |
-
-
37052-78-1
2-Mercapto-5-methoxybenzimidazole

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
73590-85-9
6-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)thio)-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 80℃; for 1h; Solvent; Temperature; | 97% |
| With sodium hydroxide In methanol; water at 55 - 65℃; for 2h; Large scale; | 86% |
| With sodium hydrogencarbonate In ethanol Reagent/catalyst; Temperature; Solvent; Reflux; Large scale; | 85.3% |
| With sodium hydroxide In ethanol; water Solvent; Reflux; Large scale; | 81.1% |
| With sodium hydroxide In methanol at 50℃; for 4h; Reagent/catalyst; Temperature; Solvent; | 34.1 g |
-
-
843615-24-7
4-(2-mercapto-5-methoxy-benzimidazole-1-sulfonyl)-3-methoxy-benzoic acid 2-(toluene-4-sulfonyl)ethyl ester

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
651729-67-8
4-methoxy-3-[5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)-benzimidazole-1-sulfonyl]-benzoic acid 2-(toluene-4-sulfonyl)ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 2h; | 96% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 2h; | 96% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
37052-78-1
6-methoxy-1H-benzoimidazole-2-thiol

-
-
73590-58-6
omeprazole

| Conditions | Yield |
|---|---|
| Stage #1: 6-methoxy-1H-benzoimidazole-2-thiol With sodium hydroxide In ethanol; water at 70 - 90℃; Stage #2: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride With hydrogenchloride In ethanol; water at -10 - 30℃; for 4h; Reflux; | 96% |
-
-
129422-96-4
1-(p-toluenesulfonyl)-1,4,7-triazacyclononane

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium carbonate In acetonitrile for 24h; Inert atmosphere; Reflux; | 96% |
| With tetrabutylammomium bromide; sodium carbonate In acetonitrile for 22h; Reflux; Inert atmosphere; | 91% |
-
-
37052-78-1
5-methoxy-1,3-dihydro-2H-benzo[d]imidazole-2-thione

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
73590-85-9
omeprazole sulfide

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol for 1.5h; Reflux; | 95% |
| In methanol at 70℃; Kinetics; |
-
-
136247-90-0
tert-butyl (3-(methoxymethoxy)phenyl)carbamate

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (3-(methoxymethoxy)phenyl)carbamate With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 0.5h; Stage #2: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 95% |
-
-
97963-62-7
5-(difluoromethoxy)-2-mercapto-1H-benzimidazole

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
102625-64-9
pantoprazole sulfide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 80℃; for 8h; | 94% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
164513-38-6
2-hydroxy-4-methyl-5-bromopyridine

| Conditions | Yield |
|---|---|
| With silver carbonate In toluene at 110℃; for 6h; Darkness; | 94% |

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
651729-45-2
2-(4-tolylsulfonyl)ethyl 3-{4-[(5-methoxy-2-{[4-methoxy-3,5-dimethyl(2-pyridyl)]methylthio}benzimidazolyl)sulfonyl]-3,5-dimethylphenoxy}-2,2-dimethylpropionate

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4-tolylsulfonyl)ethyl 3-{4-[2-mercapto-5-methoxybenzimidazolyl]sulfonyl}-3,5-dimethylphenoxy-2,2-dimethylpropionate; 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride With potassium carbonate In DMF (N,N-dimethyl-formamide) for 1.5h; Stage #2: With hydrogenchloride at 0℃; | 93% |
-
-
651729-74-7
3-[4-(2-mercapto-5-methoxy-benzimidazole-1-sulfonyl)-phenoxy]-2,2-dimethyl-propionic acid methyl ester

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
651729-75-8
3-[4-(5-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)benzimidazole-1-sulfonyl)-phenoxy]-2,2-dimethyl-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 1h; | 93% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 1h; | 93% |
-
-
843615-34-9
2-carbamoylmethoxy-4-[{6-methoxy-2-mercaptobenzimidazolyl}sulfonyl]phenoxyacetamide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
843615-35-0
1-(3,4-dimethoxycarboxamidobenzenesulfonyl)-6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]thio-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 0.25h; | 93% |
-
-
1319671-99-2
C14H18N4OS

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In ethanol; acetone at 45℃; for 4h; | 92% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium carbonate In acetonitrile at 80℃; for 48h; Inert atmosphere; | 92% |
| With potassium carbonate In acetonitrile for 72h; Inert atmosphere; Reflux; | 49% |
| Inert atmosphere; Reflux; |
-
-
54923-31-8
3-bromo-6-hydroxy-2-methylpyridine

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

| Conditions | Yield |
|---|---|
| With silver carbonate In toluene at 110℃; for 6h; Darkness; | 92% |
-
-
1074-82-4
potassium phtalimide

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
946071-49-4
2-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 85℃; Inert atmosphere; | 91% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
848694-10-0
2-chloromethyl-3,5-dimethyl-4-methoxypyridine-N-oxide

| Conditions | Yield |
|---|---|
| Stage #1: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride With sodium hydroxide In chloroform for 0.5h; Stage #2: With 3-chloro-benzenecarboperoxoic acid In chloroform at 55 - 60℃; for 1.5h; Further stages.; | 90.4% |
| Stage #1: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride With sodium hydrogencarbonate In chloroform Stage #2: With 3-chloro-benzenecarboperoxoic acid at 5℃; for 2.41667h; Further stages.; | 52.47% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
134469-07-1
Benzimidazol-2-thiol

-
-
73590-87-1
2-[(3,5-dimethyl-4-methoxy-2-pyridinylmethyl)thio]-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 68℃; for 4h; | 89.5% |
| With sodium hydroxide In methanol Heating; | 84% |
-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
84445-90-9
5-acetamido-benzimidazoline-2-thione

-
-
117013-90-8
N-[2-(4-Methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)-1H-benzoimidazol-5-yl]-acetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 90℃; for 1h; | 89% |
-
-
651729-82-7
[4-(2-mercapto-6-methoxy-benzimidazole-1-sulfonyl)-phenoxy]acetic acid methyl ester

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
651729-83-8
[4-(6-methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)benzimidazole-1-sulfonyl)-phenoxy]acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 1.25h; | 89% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) for 1h; | 89% |
-
-
17536-00-4
3,5-diisopropylpyrazole

-
-
86604-75-3
2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride

-
-
1191928-12-7
2-[(3,5-diisopropyl-1H-pyrazol-1-yl)methyl]-4-methoxy-3,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; sodium hydroxide In water; toluene Inert atmosphere; Reflux; | 89% |
| With tetra(n-butyl)ammonium hydroxide; sodium hydroxide In water; toluene |
2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride Specification
The 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride, with the CAS registry number 86604-75-3, has the systematic name of 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride (1:1). It belongs to the following product categories: Heterocyclic Building Blocks; Halogenated Heterocycles; Pyridines. The molecular formula of this chemical is C9H12ClNO.HCl. What's more, it is usually used as intermediate of omeprazole.You should be cautious while dealing with 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride. It may cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
The physical properties of 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride are as following:
(1)ACD/LogP: 2.19; (2)# of Rule of 5 Violations: 0; (3)#H bond acceptors: 2; (4)#H bond donors: 0; (5)#Freely Rotating Bonds: 2; (6)Polar Surface Area: 22.12 Å2; (7)Flash Point: 118.4 °C; (8)Enthalpy of Vaporization: 48.99 kJ/mol; (9)Boiling Point: 272.2 °C at 760 mmHg; (10)Vapour Pressure: 0.0103 mmHg at 25°C.
Uses of 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride:
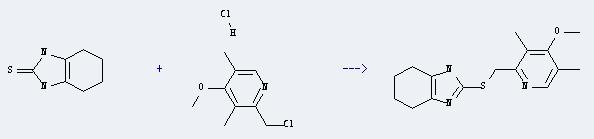
It can react with 4,5,6,7-tetrahydrobenzimidazoline-2-thione to produce 2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethylsulfanyl)-4,5,6,7-tetrahydro-1H-benzoimidazole. This reaction will need reagent NaOH, and the solvents ethanol and H2O. The reaction time is 1 hour with temperature of 90°C, and the yield is about 85%.
You can still convert the following datas into molecular structure:
(1)SMILES: ClCc1ncc(c(OC)c1C)C.Cl
(2)InChI: InChI=1/C9H12ClNO.ClH/c1-6-5-11-8(4-10)7(2)9(6)12-3;/h5H,4H2,1-3H3;1H
(3)InChIKey: LCJDHJOUOJSJGS-UHFFFAOYAO
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View