-
Name
Isopropyl chloride
- EINECS 200-858-8
- CAS No. 75-29-6
- Article Data189
- CAS DataBase
- Density 0.874 g/cm3
- Solubility 3.1 g/L (20 °C) in water
- Melting Point -118 °C
- Formula C3H7Cl
- Boiling Point 37.254 °C at 760 mmHg
- Molecular Weight 78.5416
- Flash Point -35 °C
- Transport Information UN 2456 3/PG 1
- Appearance colorless liquid with pleasant smells
- Safety 9-29
- Risk Codes 12-36/37-20/21/22-11
-
Molecular Structure
-
Hazard Symbols
 F+,
F+,  Xi,
Xi,  Xn,
Xn,  F
F
- Synonyms 2-Propyl chloride;Chlorodimethylmethane;Isoprid;Isopropyl chloride;Propane, 2-chloro-;Narcosop;R 280da;
- PSA 0.00000
- LogP 1.63360
Synthetic route

| Conditions | Yield |
|---|---|
| With indium(III) chloride; dimethylmonochlorosilane; benzil In dichloromethane at 20℃; for 6h; | 100% |
| With priphenylchlorophosphonium phosphorodichloridate at 20℃; Arbuzov reaction; | 84% |
| With hydrogenchloride at 120℃; under 7500.75 Torr; Flow reactor; | 54% |
-
-
74-93-1
methylthiol

-
-
1912-24-9
6-chloro-N-ethyl-N'-isopropyl-1,3,5-triazine-2,4-diamine

-
A
-
75-29-6
isopropyl chloride

-
B
-
834-12-8
ametryn

| Conditions | Yield |
|---|---|
| With ZSM-5 In isopropyl alcohol at 90 - 110℃; under 3675.37 - 5850.59 Torr; for 1h; Temperature; Pressure; Reagent/catalyst; Sealed tube; | A 70.91 g B 99.9% |


| Conditions | Yield |
|---|---|
| With thionyl chloride In tetrachloromethane for 2h; Heating; | A 99% B n/a |
-
-
143885-03-4
isopropyloxy(diphenyl)-λ6-sulfanenitrile

-
A
-
75-29-6
isopropyl chloride

-
B
-
22731-83-5
S,S-diphenylsulphoximine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In [D3]acetonitrile at 20℃; for 0.25h; | A 99% B n/a |
-
-
110-86-1
pyridine

-
A
-
75-29-6
isopropyl chloride

-
B
-
1395411-06-9
cis-Cl(py)Pt(COMe)[C(OiPr)(Me)]

-
C
-
67-63-0
isopropyl alcohol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 at 55℃; for 21h; | A n/a B 98% C n/a |

-
-
187737-37-7
propene

-
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; chromium; silica gel at 140℃; Product distribution; Rate constant; oth. temperature, oth. catalysts, E(activ.); | 95% |
| With hydrogenchloride; aluminium oxide, chlorided at 40 - 60℃; under 759.826 - 1519.65 Torr; for 200h; | 94% |
| With hydrogenchloride; aluminium oxide, fluorided for 200h; | 94% |
-
-
56-23-5
tetrachloromethane

-
-
80937-33-3
oxygen

-
-
1071-39-2
diisopropylmercury

-
A
-
74-98-6
propane

-
B
-
75-29-6
isopropyl chloride

-
C
-
67-66-3
chloroform

-
D
-
30615-19-1
isopropylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) 20°C, 96 h; further products; | A 4% B 48% C 30% D 95% E 5% |


| Conditions | Yield |
|---|---|
| With tin(IV) chloride In dichloromethane at 110℃; for 60h; sealed tube; Inert atmosphere; | 95% |
| With titanium tetrachloride In dichloromethane at 150℃; for 60h; |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 0 - 50℃; for 50h; | A 94% B n/a |
-
-
187737-37-7
propene

-
A
-
557-98-2
2-chloropropene

-
B
-
26198-63-0, 78-87-5
1,2-Dichloropropane

-
C
-
542-75-6
E/Z-1,3-Dichloropropene

-
D
-
75-29-6
isopropyl chloride

-
E
-
590-21-6
propenyl chloride

-
F
-
107-05-1
3-chloroprop-1-ene

| Conditions | Yield |
|---|---|
| With aluminum oxide; copper dichloride; dibenzoyl peroxide at 489.9℃; for 0.000555556h; Product distribution; Mechanism; also other temperatures (503 K - 753 K) and initiators (chloral dioxyperoxide); | A 0.7% B 3.3% C 0.8% D 1.8% E 0.9% F 91.4% |

-
-
56-23-5
tetrachloromethane

-
-
1071-39-2
diisopropylmercury

-
B
-
75-29-6
isopropyl chloride

-
C
-
67-66-3
chloroform

-
D
-
30615-19-1
isopropylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) 130°C, 10 h; further products; | A 3% B 51% C 50% D 91% E 3% |

-
-
116-17-6
triisopropyl phosphite

-
-
40510-88-1
1-acetoxy-2-chloromethoxyethane

-
A
-
75-29-6
isopropyl chloride

-
B
-
162612-58-0
diisopropyl <(2-acetoxyethoxy)methyl>phosphonate

| Conditions | Yield |
|---|---|
| at 110℃; for 3h; Inert atmosphere; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In dichloromethane at 0℃; | A n/a B 84% |

| Conditions | Yield |
|---|---|
| With zinc(II) phosphide; oxygen; copper dichloride at 50℃; under 750.06 Torr; Title compound not separated from byproducts; | A n/a B 82.3% |
| With zinc(II) phosphide; oxygen; copper dichloride at 50℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With CPT | A n/a B 74% |

| Conditions | Yield |
|---|---|
| With dichloromethane; antimony pentafluoride at -78 - 20℃; Product distribution; Mechanism; other alkanes and cycloalkanes; methylene bromide; | 69% |
| With dichloromethane; antimony pentafluoride 1.) -78 deg C, 2 h, 2.) RT, 24 h; | 69% |
| In various solvent(s) at -158.1℃; Kinetics; Irradiation; |

| Conditions | Yield |
|---|---|
| With pyrographite at -196.16℃; under 1E-05 - 0.0001 Torr; | A 66% B 34% |
-
-
2176-62-7
2,3,4,5,6-pentachloropyridine

-
-
116-17-6
triisopropyl phosphite

-
A
-
75-29-6
isopropyl chloride

-
B
-
87361-54-4
2,3,5,6-Tetrachlor-4-pyridylphosphonsaeure-diisopropylester

| Conditions | Yield |
|---|---|
| at 130℃; for 16h; | A n/a B 60% |

-
-
116-17-6
triisopropyl phosphite

-
-
57182-16-8
2,2,2-trichloro-N-methylacetimidoyl chloride

-
A
-
75-29-6
isopropyl chloride

-
B
-
70795-54-9
C15H31Cl2NO6P2

| Conditions | Yield |
|---|---|
| In toluene for 2h; Heating; | A n/a B 56% |

-
-
116-17-6
triisopropyl phosphite

-
-
57182-25-9
N-ethyl-2,2,2-trichloro-acetimidoyl chloride

-
A
-
75-29-6
isopropyl chloride

-
B
-
70795-58-3
{2,2-Dichloro-1-[(diisopropoxy-phosphoryl)-ethyl-amino]-vinyl}-phosphonic acid diisopropyl ester

| Conditions | Yield |
|---|---|
| In toluene for 2h; Heating; | A n/a B 51% |

-
-
187737-37-7
propene

-
-
75-09-2
dichloromethane

-
A
-
75-29-6
isopropyl chloride

-
B
-
1190-22-3
1,3-dichlorobutane

-
C
-
616-19-3
1,3-dichloro-2-methylpropane

| Conditions | Yield |
|---|---|
| With RhCl(PPh3)3 at 200℃; for 3h; | A 50% B 23% C 15% |

-
-
187737-37-7
propene

-
-
67-66-3
chloroform

-
A
-
75-29-6
isopropyl chloride

-
B
-
66675-32-9
1,1,2-trichloro-butane

-
C
-
62108-65-0
1,1,3-trichloro-2-methyl-propane

| Conditions | Yield |
|---|---|
| With RhCl(PPh3)3 at 200℃; for 3h; | A 46% B 20% C 12% |


-
A
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| at 170℃; under 0.03 Torr; | A 20% B 31% |
| at 165 - 170℃; under 0.03 Torr; Product distribution; |
-
-
594-20-7
2,2-dichloropropane

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
557-98-2
2-chloropropene

-
C
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| With iron pentacarbonyl at 140℃; for 3h; Product distribution; various reaction conditions; | A 8% B 5% C 16% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminum oxide at 420℃; |

| Conditions | Yield |
|---|---|
| With mercury dichloride |
-
-
56-23-5
tetrachloromethane

-
-
74-98-6
propane

-
-
110-05-4
di-tert-butyl peroxide

-
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| at 140℃; |

-
-
56-23-5
tetrachloromethane

-
-
74-98-6
propane

-
-
110-05-4
di-tert-butyl peroxide

-
A
-
75-29-6
isopropyl chloride

-
B
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| at 138℃; |

-
-
56-23-5
tetrachloromethane

-
-
3437-84-1
isobutyroyl peroxide

-
A
-
75-29-6
isopropyl chloride

-
B
-
67-72-1
hexachloroethane

-
-
98070-46-3
trichlorophosphoranylidene-carbamic acid isopropyl ester

-
A
-
870-30-4
isocyanatophosphonic dichloride

-
B
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| Zersetzt; |
-
-
75-29-6
isopropyl chloride

-
-
1000993-74-7
1-(4-isopropyl-5-methyl-2-furyl)propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 5-methyl-2-propionylfuran; aluminum (III) chloride at 0 - 30℃; for 0.5h; Stage #2: isopropyl chloride at 0 - 10℃; | 100% |
-
-
1020552-35-5, 59461-43-7, 103364-89-2
(1R,4S,8R,11S)-(1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane)nickel(I)

-
-
75-29-6
isopropyl chloride

-
-
103258-96-4
(1R,4S,8R,11S)-(1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane)(2-propyl)nickel(II)

| Conditions | Yield |
|---|---|
| In water Kinetics; reaction of R,S,R,S-Ni(tmc)(1+) with 2-PrCl at 25°C under Cr(2+)-scrubbed Ar; | 100% |
-
-
75-29-6
isopropyl chloride

-
-
1051899-64-9
N2-(3,5-dimorpholinophenyl)-N2-(4-methoxybenzyl)-N4-(3-methoxyphenyl)pyrimidine-2,4-diamine

-
-
1051899-65-0
N2-(3,5-dimorpholinophenyl)-N4-isopropyl-N2-(4-methoxybenzyl)-N4-(3-methoxyphenyl)pyrimidine-2,4-diamine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 25 - 90℃; for 21h; | 100% |
-
-
10599-69-6
5-methyl-2-propionylfuran

-
-
75-29-6
isopropyl chloride

-
-
1000993-74-7
1-(4-isopropyl-5-methyl-2-furyl)propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 5-methyl-2-propionylfuran With aluminum (III) chloride at 0 - 30℃; for 0.5h; Neat (no solvent); Stage #2: isopropyl chloride at 0 - 10℃; Product distribution / selectivity; Neat (no solvent); | 100% |
| Stage #1: 5-methyl-2-propionylfuran With aluminum (III) chloride In dichloromethane at 0 - 35℃; for 0.5h; Stage #2: isopropyl chloride In dichloromethane at 0 - 10℃; for 1h; Industry scale; Stage #3: With sodium hydroxide; water at 0℃; pH=1; Product distribution / selectivity; | 100% |
| Stage #1: 5-methyl-2-propionylfuran With aluminum (III) chloride at 0 - 30℃; for 0.5h; Stage #2: isopropyl chloride at 0 - 10℃; Stage #3: With water at 0℃; |
-
-
75-29-6
isopropyl chloride

-
-
151266-23-8
4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidine

-
-
862730-04-9
3-iodo-1-(propan-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 200℃; for 0.0833333h; Microwave irradiation; regioselective reaction; | 100% |
-
-
75-29-6
isopropyl chloride

-
-
14143-26-1
4-hydroxy-2-methylbenzonitrile

| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide; potassium carbonate In toluene at 25 - 55℃; for 10h; Reagent/catalyst; Temperature; | 98.9% |
-
-
75-29-6
isopropyl chloride

-
-
111757-80-3
(-)-(2S)-trifluoromethanesulfonyloxy-propionic acid tert-butyl ester

-
-
1059043-99-0
(+)-(2S,3)-dimethyl-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl chloride With magnesium In tetrahydrofuran Inert atmosphere; Stage #2: (-)-(2S)-trifluoromethanesulfonyloxy-propionic acid tert-butyl ester With zinc(II) chloride In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; optical yield given as %ee; enantiospecific reaction; | 98% |
-
-
17771-33-4
7-hydroxy-2,2-dimethyl-chroman-4-one

-
-
75-29-6
isopropyl chloride

-
-
120046-15-3
7-Isopropoxy-2,2-dimethyl-chroman-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 80℃; for 12h; | 96% |
-
-
75-29-6
isopropyl chloride

-
-
85417-41-0
tris(2,6-dimethoxyphenyl)phosphine

| Conditions | Yield |
|---|---|
| With perchloric acid In ethanol at 65℃; for 15h; | 96% |

| Conditions | Yield |
|---|---|
| With AlCl3 In tetrahydrofuran under N2; pinch of AlCl3 added to the soln. of the Ta-complex in THF, addn. of excess i-PrCl at room temp., soln. stirred (8 h); removal of volatiles (vac.), elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl chloride; zinc(II) chloride In tetrahydrofuran at 20℃; for 1h; Stage #2: 1-phenyl-propan-1-one In tetrahydrofuran at 0℃; for 2h; | 95% |
| (i) Mg, I2, Et2O, (ii) /BRN= 606215/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride In dichloromethane at 23℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| With magnesium In tetrahydrofuran-d8 at 80℃; under 5171.62 Torr; Temperature; Concentration; Inert atmosphere; Flow reactor; | 95% |
| With magnesium In tetrahydrofuran at 21 - 29℃; for 0.5h; Inert atmosphere; Flow reactor; | 94.5% |
| With magnesium In tetrahydrofuran |
-
-
75-29-6
isopropyl chloride

-
-
91608-15-0
tris(2,4,6-trimethoxyphenyl)phosphine

| Conditions | Yield |
|---|---|
| With perchloric acid In ethanol at 65℃; for 15h; | 95% |
-
-
75-29-6
isopropyl chloride

-
-
19722-76-0
methyl-2,6-dihydroxy-4-methoxybenzoate

-
-
231621-29-7
2-fluoro-6-methoxy-3-pivaloylbenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl chloride; 2-fluoro-6-methoxy-3-pivaloylbenzonitrile With antimonypentachloride In dichloromethane for 0.666667h; Stage #2: methyl-2,6-dihydroxy-4-methoxybenzoate In dichloromethane at 20℃; for 2h; Further stages.; | 95% |


-
-
75-29-6
isopropyl chloride

-
-
140-53-4
p-chlorobenzyl cyanide

-
-
2012-81-9
3-Methyl-2-(4'-chlorophenyl)-butyronitrile

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 5,5-dimethyl-1,3-cyclohexadiene; water | 95% |

-
-
75-29-6
isopropyl chloride

-
-
151266-23-8
4-amino-3-iodo-pyrazolo[3,4-d]pyrimidine

-
-
862730-04-9
3-iodo-1-(propan-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 200℃; Microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; C21H23ClNiP2S; caesium carbonate; sodium iodide In dimethyl sulfoxide at 50℃; for 12h; Sonogashira Cross-Coupling; Schlenk technique; Inert atmosphere; | 95% |
-
-
75-29-6
isopropyl chloride

-
-
156839-06-4
2-methyldithiocarbazic acid isopropyl ester

| Conditions | Yield |
|---|---|
| 94% | |
| 94% |
-
-
75-29-6
isopropyl chloride

-
-
138526-69-9
3,4,5-trifluoro-1-bromobenzene

-
-
1310112-87-8
1-bromo-3,4,5-trifluoro-2,6-diisopropylbenzene

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at 0℃; for 3h; Inert atmosphere; | 94% |
| aluminum (III) chloride at 20℃; for 2h; Inert atmosphere; |
-
-
75-29-6
isopropyl chloride

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In 1,4-dioxane at 100℃; for 3h; Inert atmosphere; Sealed tube; | 94% |
2-Chloropropane Standards and Recommendations
2-Chloropropane Specification
2-Chloropropane is an organic compound with the formula C3H7Cl, and its systematic name is the same with the product name. With the CAS registry number 75-29-6, it is also named as Isopropyl chloride. It belongs to the product categories of Organics; Chloro Alkane Compounds. Its EINECS number is 209-187-5. In addition, the molecular weight is 78.54. Its classification code is Mutation data. This chemcial should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from light, heat and fire. This chemical is used as raw material in organic synthetic to prepare pesticide propachlor. It is also used as a solvent.
Physical properties of 2-Chloropropane are: (1)ACD/LogP: 1.976; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.98; (4)ACD/LogD (pH 7.4): 1.98; (5)ACD/BCF (pH 5.5): 18.70; (6)ACD/BCF (pH 7.4): 18.70; (7)ACD/KOC (pH 5.5): 283.14; (8)ACD/KOC (pH 7.4): 283.14; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Index of Refraction: 1.379; (13)Molar Refractivity: 20.755 cm3; (14)Molar Volume: 89.817 cm3; (15)Polarizability: 8.228×10-24cm3; (16)Surface Tension: 18.92 dyne/cm; (17)Density: 0.874 g/cm3; (18)Flash Point: -35 °C; (19)Enthalpy of Vaporization: 27.037 kJ/mol; (20)Boiling Point: 37.254 °C at 760 mmHg; (21)Vapour Pressure: 488.79 mmHg at 25°C.
Preparation of 2-Chloropropane: this chemical can be prepared by propan-2-ol at the temperature 20 °C. This reaction will need reagent chlorotriphenylphosphonium dichlorophosphate. The yield is about 84%.

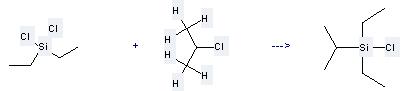
Uses of 2-Chloropropane: it can be used to produce chlorodiethylisopropylsilane at the temperature of 50 °C. It will need reagent Li and solvent petroleum ether with the reaction time of 1 hour. The yield is about 92%.
When you are using this chemical, please be cautious about it as the following:
This chemcial is extremely flammable, so you should keep it away from sources of ignition - No smoking. It is harmful by inhalation, in contact with skin and if swallowed. This substance is irritating to eyes and respiratory system. You should keep the container in a well-ventilated place, and you must not empty it into drains.
You can still convert the following datas into molecular structure:
(1)SMILES: ClC(C)C
(2)Std. InChI: InChI=1S/C3H7Cl/c1-3(2)4/h3H,1-2H3
(3)Std. InChIKey: ULYZAYCEDJDHCC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 119gm/m3 (119000mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 55(7), Pg. 93, 1990. | |
| mouse | LD50 | oral | 1300mg/kg (1300mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC LUNGS, THORAX, OR RESPIRATION: CYANOSIS | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 55(7), Pg. 93, 1990. |
| rat | LC50 | inhalation | 120gm/m3 (120000mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 55(7), Pg. 93, 1990. | |
| rat | LC50 | inhalation | 120gm/m3 (120000mg/m3) | BLOOD: CHANGES IN LEUCOCYTE (WBC) COUNT | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 55(7), Pg. 93, 1990. |
| rat | LD50 | oral | 5gm/kg (5000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC LUNGS, THORAX, OR RESPIRATION: CYANOSIS | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 55(7), Pg. 93, 1990. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View