 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
2-Diisopropylaminoethanol
- EINECS 202-536-2
- CAS No. 96-80-0
- Article Data7
- CAS DataBase
- Density 0.826
- Solubility
- Melting Point -39.3ºC
- Formula C8H19 N O
- Boiling Point 187-192 ºC
- Molecular Weight 145.245
- Flash Point 158 ºF
- Transport Information UN 2825/2922
- Appearance colourless to slightly yellow liquid
- Safety 26-36/37/39-45
- Risk Codes R20/22;R24;R34
-
Molecular Structure
-
Hazard Symbols

- Synonyms Ethanol,2-(diisopropylamino)- (6CI,7CI,8CI); (Diisopropylamino)ethanol;(N,N-Diisopropylamino)ethanol; 2-(Diisopropylamino)ethanol;2-(Diisopropylamino)ethyl alcohol; 2-(N,N-Diisopropylamino)ethanol;Diisopropylethanolamine; N,N-Diisopropyl-2-aminoethanol;N,N-Diisopropylethanolamine; NSC 45475
- PSA 23.47000
- LogP 1.09750
Synthetic route

| Conditions | Yield |
|---|---|
| at 200℃; | |
| at 200℃; |

| Conditions | Yield |
|---|---|
| at 120℃; | |
| In toluene for 3h; Reflux; |
-
-
75-21-8
oxirane

-
-
108-18-9
diisopropylamine

-
A
-
96-80-0
2-(diisopropylamino)ethanol

-
B
-
17844-23-4
4-methylene-hex-5-en-1-ol

| Conditions | Yield |
|---|---|
| With n-butyllithium; potassium tert-butylate; isoprene 1.) THF/hexane, -70 deg C, 1 min 2.) THF/hexane, -70 deg C to -20 deg C; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
-
-
96-80-0
2-(diisopropylamino)ethanol

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 80℃; for 10h; Large scale; | 93% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
108-18-9
diisopropylamine

-
-
74-86-2
acetylene

-
-
52183-40-1
bis(2-diisopropylaminoethyl) ether

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,3-dioxane at 120℃; for 3h; | 85.6% |

-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
144191-92-4
(11-hydroxyundecyl)carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (11-hydroxyundecyl)carbamic acid tert-butyl ester With dmap; triethylamine; methylphosphonothioic dichloride In chloroform at 20℃; for 3h; Inert atmosphere; Stage #2: 2-(diisopropylamino)ethanol With triethylamine In chloroform at 20℃; for 16h; Inert atmosphere; | 82% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
96-79-7
(2-chloroethyl)-diisopropylamine

-
-
52183-40-1
bis(2-diisopropylaminoethyl) ether

| Conditions | Yield |
|---|---|
| With sodium hydroxide; adogen 464 In tetrahydrofuran for 6h; Heating; | 74% |
-
-
864757-74-4
4-(benzyloxy)-1-(4-hydroxyphenyl)pyridin-2(1H)-one

-
-
96-80-0
2-(diisopropylamino)ethanol

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 4h; Mitsunobu reaction; | 73% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
100-00-5
4-chlorobenzonitrile

-
-
85002-94-4
Diisopropyl-[2-(4-nitro-phenoxy)-ethyl]-amine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide at 70℃; for 0.5h; | 67.2% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
193966-49-3
4-fluorophenyl 2-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]benzo[b]thiophen-3-yl ketone

-
-
193966-53-9
(4-[2-(diisopropylamino)ethoxy]phenyl)-(2-[4-[2-(1-pyrrolidinyl)ethoxy]-phenyl]benzo[b]thiophen-3-yl)ketone

| Conditions | Yield |
|---|---|
| Stage #1: 2-(diisopropylamino)ethanol With sodium hydride In N,N-dimethyl-formamide for 0.25h; deprotonation; Stage #2: 4-fluorophenyl 2-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]benzo[b]thiophen-3-yl ketone In N,N-dimethyl-formamide at 20℃; for 5h; Condensation; Further stages.; | 62% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
74-88-4
methyl iodide

-
-
139709-38-9
N,N-diisopropyl-N-(2-methoxyethyl)amine

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 40℃; for 0.5h; | 58% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
167940-05-8
N,N-diisopropyl-(2-thiocyanatoethyl)amine

| Conditions | Yield |
|---|---|
| Stage #1: lead thiocyanate With bromine In acetonitrile at -40℃; for 0.5h; Stage #2: With triphenylphosphine In acetonitrile at -40℃; for 0.5h; Stage #3: 2-(diisopropylamino)ethanol In acetonitrile at -40℃; for 2h; Further stages.; | 56% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
76-05-1
trifluoroacetic acid

-
-
480445-51-0
8-(4-bromo-benzyl)-3-isobutyl-3,7-dihydro-purine-2,6-dione

| Conditions | Yield |
|---|---|
| Stage #1: 8-(4-bromo-benzyl)-3-isobutyl-3,7-dihydro-purine-2,6-dione With {4-[(2,4-dimethoxyphenyl)chloromethyl]phenoxy}-Rink resin; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 6h; Stage #2: 2-(diisopropylamino)ethanol With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 1h; Mitsunobu reaction; Stage #3: trifluoroacetic acid In dichloromethane at 20℃; for 0.5h; | 45% |

-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
626-15-3
1,3-bis-(bromomethyl)benzene

-
-
1246680-25-0
N-(2-(3-(bromomethyl)benzyloxy)ethyl)-N-isopropylpropan-2-amine

| Conditions | Yield |
|---|---|
| Stage #1: 2-(diisopropylamino)ethanol With n-butyllithium; potassium iodide In tetrahydrofuran; hexane at -78℃; for 0.166667h; Inert atmosphere; Stage #2: 1,3-bis-(bromomethyl)benzene In tetrahydrofuran; hexane at -78 - 75℃; Inert atmosphere; | 45% |
-
-
179942-97-3
7-bromo-1-cyclohexyl-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid

-
-
96-80-0
2-(diisopropylamino)ethanol

| Conditions | Yield |
|---|---|
| 38% |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
848472-41-3
N-{2-[(6-chloropyrimidin-4-yl)oxy]ethyl}-N-isopropylpropan-2-amine

| Conditions | Yield |
|---|---|
| With caesium carbonate In propyl cyanide at 180℃; for 0.333333h; microwave irradiation; | 20% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
204758-33-8
4-(2-chloroethyl)bis[1,2]dithiolo[3,4-b:4',3'-e][1,4]thiazine-3,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: 2-(diisopropylamino)ethanol With 1,4-diaza-bicyclo[2.2.2]octane; disulfur dichloride In 1,2-dichloro-ethane at -40 - 20℃; Addition; Stage #2: With formic acid In 1,2-dichloro-ethane for 1h; Cyclization; Heating; | 13% |

| Conditions | Yield |
|---|---|
| With NH4SCN In methanol NH4SCN in MeOH added to 2-diisopropylaminoethanol and Cu(II) in MeOH, standed (5°C, several days); filtered, dried (in vac. over P2O5); elem. anal.; | 8% |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
204758-35-0
5,6-dihydro-4-(3-thiono[1,2]dithiol-4-yl)-[1,2]dithiolo[3,4-b][1,4]thiazine-3-thione

| Conditions | Yield |
|---|---|
| Stage #1: 2-(diisopropylamino)ethanol With disulfur dichloride In tetrahydrofuran at -40 - 20℃; Addition; Stage #2: With diphosphorus pentasulfide In tetrahydrofuran for 5.5h; Cyclization; Heating; | 7% |
-
-
74-83-9
methyl bromide

-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
866-67-1
(2-hydroxy-ethyl)-diisopropyl-methyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| With butanone |
-
-
78-10-4
tetraethoxy orthosilicate

-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
18642-90-5
silicic acid diethyl ester-bis-(2-diisopropylamino-ethyl ester)
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
6292-88-2
N,N-dicyclohexylcarbamoyl chloride

-
-
102444-91-7
dicyclohexyl-carbamic acid-(2-diisopropylamino-ethyl ester)

| Conditions | Yield |
|---|---|
| With toluene |

| Conditions | Yield |
|---|---|
| With thionyl chloride; chloroform | |
| With thionyl chloride; benzene | |
| With thionyl chloride Chlorination; Vogel method; | |
| With thionyl chloride In chloroform Reflux; Cooling with ice; |

| Conditions | Yield |
|---|---|
| With thionyl chloride; benzene | |
| With thionyl chloride for 3h; Heating; | |
| With thionyl chloride In toluene for 2h; Reflux; | |
| With thionyl chloride In chloroform at 0℃; for 4h; Reflux; |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
100119-45-7
4-phenyltetrahydropyran-4-carbonyl chloride

-
-
102371-15-3
4-phenyl-tetrahydro-pyran-4-carboxylic acid-(2-diisopropylamino-ethyl ester)

| Conditions | Yield |
|---|---|
| With benzene |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
853919-58-1
cyclohex-2-enyl-[2]thienyl-acetyl chloride

-
-
102370-98-9
cyclohex-2-enyl-[2]thienyl-acetic acid-(2-diisopropylamino-ethyl ester)

| Conditions | Yield |
|---|---|
| With triethylamine; benzene |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
1503-19-1
9H-Thioxanthene-9-acetic acid

-
-
113649-39-1
thioxanthen-9-yl-acetic acid-(2-diisopropylamino-ethyl ester); hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; benzene |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
100954-91-4
xanthen-9-yl-acetyl chloride

| Conditions | Yield |
|---|---|
| With benzene |
-
-
96-80-0
2-(diisopropylamino)ethanol

-
-
40020-65-3
9H-Thioxanthene-9-acetic acid 10,10-dioxide

-
-
102762-88-9
(10,10-dioxo-10λ6-thioxanthen-9-yl)-acetic acid-(2-diisopropylamino-ethyl ester); hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; benzene |

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| mit Hilfe von Silberchlorid; |
2-Diisopropylaminoethanol Chemical Properties
Molecular Formula: C8H19NO
Molar mass: 145.2426 g/mol
EINECS: 202-536-2
Density: 0.874 g/cm3
Flash Point: 60.1 °C
Index of Refraction: 1.445
Boiling Point: 189.5 °C at 760 mmHg
Vapour Pressure: 0.155 mmHg at 25°C
Water solubility: Slightly soluble in water
Appearance: Colourless to slightly yellow liquid
Stable: Stable. Incompatible with acids, strong oxidizing agents.
Structure of 2-Diisopropylaminoethanol (96-80-0):
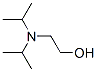
XLogP3-AA: 1.2
H-Bond Donor: 1
H-Bond Acceptor: 2
IUPAC Name: 2-[Di(propan-2-yl)amino]ethanol
Canonical SMILES: CC(C)N(CCO)C(C)C
InChI: InChI=1S/C8H19NO/c1-7(2)9(5-6-10)8(3)4/h7-8,10H,5-6H2,1-4H3
InChIKey: ZYWUVGFIXPNBDL-UHFFFAOYSA-N
2-Diisopropylaminoethanol Uses
2-Diisopropylaminoethanol (96-80-0) can be used in organic synthesis and also be used for fiber additives, emulsifiers and catalysts. In addition, it is the intermediate of anti-cholinergic drugs Propantheline bromide .
2-Diisopropylaminoethanol Production
2-Diisopropylaminoethanol (96-80-0) can be obtained from the two derived from Isopropylamine and Ethylene oxide by addition.First, join Isopropylamine and water into reaction pot, and cooled to 0-5°C, then leads to Ethylene oxide. Naturally heated to 20-30 ℃, stirring the tank reactor 4h and then gradually warmed up to 40-50°C, and then continue to respond 4h. Place overnight. The next day, the reaction solution has fractionation at atmospheric pressure to sub-to low-boiling below 90°C (recovery of applied), and below 120°C following the boiling (recovered apply), and then has the vacuum distillation for collecting 90-100°C (2.67kPa) fraction.It shall be the product. Consumption of raw materials fixed: Diisopropylamine 888kg / t, Ethylene oxide 293kg / t.
2-Diisopropylaminoethanol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 1661mg/m3/6H (1661mg/m3) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 216, 1992. | |
| mouse | LD50 | oral | 770mg/kg (770mg/kg) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 216, 1992. | |
| rabbit | LD50 | skin | 450uL/kg (0.45mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. | |
| rat | LC50 | inhalation | 1965mg/m3/6H (1965mg/m3) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 216, 1992. | |
| rat | LD50 | oral | 860mg/kg (860mg/kg) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 216, 1992. |
2-Diisopropylaminoethanol Consensus Reports
Reported in EPA TSCA Inventory.
2-Diisopropylaminoethanol Safety Profile
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant.
Hazard Codes: T![]()
Risk Statements:
20: Harmful by inhalation
22: Harmful if swallowed
24: Toxic in contact with skin
34: Causes burns
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
2-Diisopropylaminoethanol Specification
2-Diisopropylaminoethanol (96-80-0) also can be called (Diisopropylamino)ethanol ; Ethanol, 2-[bis(1-methylethyl)amino]- ; Ethanol, 2- (diisopropylamino)- ; N,N-diisopropyl ethanolamine ; Diisopropylethanolamine .
Related Products
- 2-Diisopropylaminoethanol
- 96801-39-7
- 96804-05-6
- 96804-38-5
- 96-81-1
- 968-21-8
- 96-82-2
- 96827-04-2
- 96827-05-3
- 96827-07-5
- 96829-58-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View