-
Name
2-Hydroxybenzimidazole
- EINECS 210-412-4
- CAS No. 615-16-7
- Article Data198
- CAS DataBase
- Density 1.252 g/cm3
- Solubility insoluble in water
- Melting Point >300 °C(lit.)
- Formula C7H6N2O
- Boiling Point 123.5 °C at 760 mmHg
- Molecular Weight 134.137
- Flash Point 42.6 °C
- Transport Information
- Appearance off-white to pale brown crystalline powder
- Safety 22-24/25-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Benzimidazolinone(6CI,7CI,8CI);1,3-Dihydro-2H-benzimidazol-2-one;1,3-Dihydro-2H-benzimidazole-2-one;1,3-Dihydrobenzimidazol-2-one;1H-Benzimidazol-2(3H)-one;2(3H)-Benzimidazolone;2(3H)-Oxobenzimidazole;2,3-Dihydro-2-oxo-1H-benzimidazole;2-Benzimidazolol;2-Benzimidazolone;N,N'-(1,2-Phenyleneurea);NSC 10383;NSC 178108;Urea,N,N'-(1,2-phenylene)-;o-Phenyleneurea;
- PSA 48.91000
- LogP 1.26850
Synthetic route
-
-
95-54-5
1,2-diamino-benzene

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 18h; | 99% |
| at 20℃; for 22h; | 96% |
| In N,N-dimethyl-formamide at 0 - 20℃; for 24h; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| In formamide at 130℃; for 0.333333h; | 99% |
| at 150 - 170℃; |

| Conditions | Yield |
|---|---|
| With 1-Methylpyrrolidine; selenium In tetrahydrofuran at 100℃; under 22800.7 Torr; for 20h; | 99% |
| With carbon dioxide; oxygen; potassium iodide; palladium(II) iodide In 1,2-dimethoxyethane at 100℃; under 45600 Torr; for 2h; | 99% |
| With 1-Methylpyrrolidine; selenium; oxygen In tetrahydrofuran 1.) 31 kg/cm2, 100 degC, 20 h, 2.) 25 degC, 1 h; | 99% |
-
-
1199-02-6
5-phenyl-3H-[1,3,4]oxadiazol-2-one

-
-
95-54-5
1,2-diamino-benzene

-
A
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
B
-
613-94-5
benzoic acid hydrazide

| Conditions | Yield |
|---|---|
| In various solvent(s) at 150℃; for 5h; | A 98% B n/a |


| Conditions | Yield |
|---|---|
| In N-methyl-acetamide; ethyl acetate | 98% |
| Multi-step reaction with 2 steps 1: benzene 2: 183 °C View Scheme | |
| Multi-step reaction with 2 steps 1: water 2: 150 °C View Scheme |

| Conditions | Yield |
|---|---|
| With tris(bis(trimethylsilyl)amido)lanthanum(III) In toluene at 80℃; for 24h; Catalytic behavior; Reagent/catalyst; Temperature; Time; Solvent; Inert atmosphere; Schlenk technique; chemoselective reaction; | 96% |
| In N,N-dimethyl acetamide for 0.183333h; Irradiation; | 82% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water at 20℃; under 7500.75 Torr; for 9h; UV-irradiation; | 96% |
| With Sn(IV)-doped DFNS supported CdSnO3 nanoparticles under 11251.1 Torr; for 1h; Kinetics; UV-irradiation; | 96% |
| With cesium fluoride In diethylene glycol dimethyl ether at 100℃; for 20h; Glovebox; Schlenk technique; | 95% |
-
-
144167-52-2
1-(N-carbethoxyimidoyl)-2-benzimidazolone

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| at 200℃; high vacuum sublimation; | 95% |
| at 200℃; Product distribution; high vacuum sublimation; or with acid; other 1-(N-carbethoxyimidoyl)-2-benzimidazolones; | 95% |
-
-
117576-29-1
(2‑aminophenyl)(1H‑benzo[d][1,2,3]triazol‑1‑yl)methanone

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With trimethylsilylazide; triethylamine; aniline In toluene at 110℃; for 1h; Sealed tube; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 135 - 140℃; for 12h; | 94% |
| With sulfated polyborate at 120℃; for 1.5h; | 94% |
| With acetic acid In tetrahydrofuran; water at 100 - 135℃; for 5h; Inert atmosphere; | 93.7% |
-
-
1711-61-1
5-(4-chlorophenyl)-3H-[1,3,4]oxadiazol-2-one

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide for 0.133333h; Irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With selenium; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine In toluene at 150℃; under 22800 Torr; for 4h; | 92% |
| With tin(IV) chloride; triethylamine; bis(triphenylphosphine)platinum(II) dichloride In 1,4-dioxane at 140℃; for 4h; | 56% |
-
-
28144-70-9
anthranilic acid amide

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With iodosylbenzene In dichloromethane at 20℃; for 2h; Hofmann rearrangement; | 92% |
| With potassium hydroxide; [bis(acetoxy)iodo]benzene In methanol at 0℃; for 1h; | 82% |

| Conditions | Yield |
|---|---|
| With tris(bis(trimethylsilyl)amido)lanthanum(III) In toluene at 80℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 16h; Irradiation; | 90% |
-
-
120811-68-9
1-benzoyl-4-(o-aminophenyl)semicarbazide

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In various solvent(s) at 150℃; for 5h; | 90% |
-
-
1202680-24-7
phenyl 4,5-dichloro-6-oxopyridazine-1(6H)-carboxylate

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In toluene for 11h; Solvent; Reflux; Green chemistry; | 90% |
-
-
83-01-2
diphenylcarbamic chloride

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide for 0.0666667h; Irradiation; | 89% |
-
-
74124-79-1
di(succinimido) carbonate

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In acetonitrile for 8h; Ambient temperature; | 88% |
-
-
95-54-5
1,2-diamino-benzene

-
-
616-38-6
carbonic acid dimethyl ester

-
A
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
B
-
3097-21-0
1,3-dimethyl-1,3-dihydrobenzimidazol-2-one

-
C
-
4760-34-3
N-methyl-1,2-phenylenediamine

| Conditions | Yield |
|---|---|
| With lead(II) nitrate at 169.85℃; for 0.5h; | A 88% B n/a C n/a |

-
-
32276-00-9
S,S"-bis(1-phenyl-1H-tetrazol-5-yl) dithiocarbonate

-
-
95-54-5
1,2-diamino-benzene

-
A
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
B
-
86-93-1
1-Phenyl-1H-tetrazole-5-thiol

| Conditions | Yield |
|---|---|
| In acetonitrile for 12h; | A 87% B n/a |

-
-
34840-26-1
1,3-dicarbethoxy-S-methylisothiourea

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 3h; Heating; | 85% |
-
-
34840-23-8
1,3-Dicarbomethoxy-S-methylisothiourea

-
-
95-54-5
1,2-diamino-benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 3h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide for 0.0666667h; Irradiation; | 81% |

| Conditions | Yield |
|---|---|
| With sodium azide; ammonium chloride; trichlorophosphate; N,N-dimethyl-formamide at 0 - 20℃; Curtius rearrangement; | 80% |
| With polymer-supported diphenylphosphoryl azide; triethylamine In benzene for 24h; Heating; | 75% |

| Conditions | Yield |
|---|---|
| With lithium bromide In 1,4-dioxane at 100 - 105℃; for 1h; | 78% |

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; isopropyl alcohol at 70℃; for 1h; | 76% |
-
-
16807-48-0
3‐acetyl‐2‐hydroxy‐6‐methyl‐4H‐pyran‐4‐one

-
-
95-54-5
1,2-diamino-benzene

-
A
-
615-15-6
2-Methyl-1H-benzimidazole

-
B
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
C
-
111008-23-2
4-(2-oxopropylidene)-1,2,4,5-tetrahydro-1H-1,5-benzodiazepin-2-one

| Conditions | Yield |
|---|---|
| In xylene Heating; 1:1 molar ratio; | A n/a B n/a C 75% |

-
-
124-38-9
carbon dioxide

-
-
13435-10-4
1,2-bis(trimethylsilylamino)benzene

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With pyridine; C14H34Cl2InNO2Si2 In toluene at 110℃; under 2280.15 Torr; for 12h; | 73% |
-
-
95-54-5
1,2-diamino-benzene

-
-
5852-49-3
5-phenyl-1,3,4-oxathiazol-2-one

-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 0.5h; Ambient temperature; | 72.5% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
85694-84-4, 86100-43-8
benzimidazolin-2-one disodium salt

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 0.5h; | 100% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
616-38-6
carbonic acid dimethyl ester

-
-
3097-21-0
1,3-dimethyl-1,3-dihydrobenzimidazol-2-one

| Conditions | Yield |
|---|---|
| With lead(II) nitrate at 199.85℃; for 20h; | 100% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
108-12-3
isopentanoyl chloride

-
-
93202-27-8
5-isovalerylbenzimidazolin-2-one

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,1,2,2-tetrachloroethylene at 90 - 100℃; for 1h; | 98% |
| With carbon disulfide; aluminium trichloride |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
79-04-9
chloroacetyl chloride

-
-
93202-41-6
5-(2-chloroethanoyl)-1,3-dihydrobenzimidazol-2-one

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,1,2,2-tetrachloroethylene at 90 - 100℃; for 1h; | 98% |
| With aluminum (III) chloride In 1,1,2,2-tetrachloroethane at 100℃; for 1h; Cooling with ice; | 93% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
161468-58-2
2,3-Dihydro-2-oxo-1H-benzimidazole-1,3-(2H)-dicarboxylic acid bis(1,1-dimethylethyl ester)

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran for 1h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In acetic acid Ambient temperature; | 98% |
| With N-chloro-succinimide In N,N-dimethyl-formamide at 20℃; for 6h; Inert atmosphere; | 97% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
122-04-3
4-nitro-benzoyl chloride

-
-
1240613-76-6
1-(4-nitrobenzoyl)-1,3-dihydrobenzimidazol-2-one

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 80℃; for 4.25h; | 98% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 5 - 25℃; for 19h; Friedel-Crafts Alkylation; Inert atmosphere; regioselective reaction; | 98% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
108-24-7
acetic anhydride

-
-
2735-73-1
1,3-diacetyl-1,3-dihydro-benzoimidazol-2-one

| Conditions | Yield |
|---|---|
| for 6h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With trichlorophosphate; phenol at 103 - 107℃; for 12h; | 97% |
| With trichlorophosphate at 100℃; for 15h; | 91% |
| With trichlorophosphate In neat (no solvent) | 80% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
15354-69-5
5,6-anhydro-1,2-O-isopropylidene-α-D-glucofuranose

-
-
869104-29-0
1,3-N,N'-bis-(6-deoxy-1,2-O-isopropylidene-α-D-glucofuranos-6-yl)benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide; toluene at 100℃; for 2h; | 97% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
83330-80-7
diethyl 2,2'-(2-oxo-1H-benzimidazole-1,3[2H]-diyl)diacetate

| Conditions | Yield |
|---|---|
| With sodium hydride In neat (no solvent) for 0.333333h; Milling; Green chemistry; | 97% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
79-30-1
isobutyryl chloride

-
-
93202-43-8
5-isobutyrylbenzimidazolin-2-one

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,1,2,2-tetrachloroethylene at 90 - 100℃; for 1h; | 96% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
161468-58-2
2,3-Dihydro-2-oxo-1H-benzimidazole-1,3-(2H)-dicarboxylic acid bis(1,1-dimethylethyl ester)

-
-
161468-45-7
2,3-Dihydro-2-oxo-1H-benzimidazole-1-carboxylic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Heating; | 96% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
74-96-4
ethyl bromide

-
-
6648-01-7
1,3-diethyl-1,3-dihydro-2H-benzimidazol-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutyl-ammonium chloride In benzene at 60℃; for 5h; | 95% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
79-03-8
propionyl chloride

-
-
93202-42-7
5-propionylbenzimidazolin-2-one

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,1,2,2-tetrachloroethylene at 90 - 100℃; for 1h; | 95% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
74-88-4
methyl iodide

-
-
3097-21-0
1,3-dimethyl-1,3-dihydrobenzimidazol-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutyl-ammonium chloride In benzene at 60℃; for 5h; | 95% |
| Stage #1: 1,3-dihydro-2H-benzimidazol-2-one With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.25h; Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 20℃; for 12h; | 83% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 79% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
1609-47-8
diethyl dicarbonate

-
-
161468-57-1
2-oxo-benzoimidazole-1,3-dicarboxylic acid diethyl ester

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran for 0.5h; | 95% |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
75-36-5
acetyl chloride

-
-
2735-73-1
1,3-diacetyl-1,3-dihydro-benzoimidazol-2-one

| Conditions | Yield |
|---|---|
| With triethylamine for 5h; Heating; | 94% |
| With pyridine |
-
-
615-16-7
1,3-dihydro-2H-benzimidazol-2-one

-
-
7325-39-5
ethyl pyridine-2-yl carbonate

-
-
41120-23-4
ethyl 2-oxo-2,3-dihydro-1H-benzo[d]imidazole-1-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 0.5h; Heating; | 94% |
| With potassium carbonate In acetonitrile for 2h; Reflux; Inert atmosphere; | 87% |
2-Hydroxybenzimidazole Consensus Reports
2-Hydroxybenzimidazole Specification
The 2-Oxobenzimidazole, with the CAS registry number 615-16-7 and EINECS registry number 210-412-4, has the systematic name of 1,3-dihydro-2H-benzimidazol-2-one. It is a kind of off-white to pale brown crystalline powder, and belongs to the following product categories: Benzimidazole; Pharmaceutical Intermediates; Benzimidazole Series; Imidazoles, Pyrroles, Pyrazoles, Pyrrolidines; Imidazol & Benzimidazole; API intermediates; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals. And the molecular formula of this chemical is C7H6N2O. What's more, it is usually used as a metabolite of the neuroleptic Droperidol.
The physical properties of 2-Oxobenzimidazole are as followings: (1)ACD/LogP: 1.12; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.12; (4)ACD/LogD (pH 7.4): 1.12; (5)ACD/BCF (pH 5.5): 4.18; (6)ACD/BCF (pH 7.4): 4.18; (7)ACD/KOC (pH 5.5): 96.89; (8)ACD/KOC (pH 7.4): 96.89; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.55 Å2; (13)Index of Refraction: 1.587; (14)Molar Refractivity: 36.03 cm3; (15)Molar Volume: 107 cm3; (16)Polarizability: 14.28×10-24cm3; (17)Surface Tension: 42.8 dyne/cm; (18)Density: 1.252 g/cm3; (19)Flash Point: 42.6 °C; (20)Enthalpy of Vaporization: 36.15 kJ/mol; (21)Boiling Point: 123.5 °C at 760 mmHg; (22)Vapour Pressure: 13.3 mmHg at 25°C.
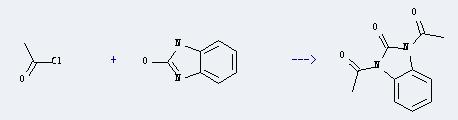
Uses of 2-Oxobenzimidazole: It can react with acetyl chloride to produce 1,3-diacetyl-1,3-dihydro-benzoimidazol-2-one. This reaction will need reagent (C2H5)3N. The reaction time is 5 hours with heating, and the yield is about 94%.
You should be cautious while dealing with this chemical. It irritates to eyes, respiratory system and skin. Therefore, you should not breathe dust and then try to avoid contacting with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C2Nc1ccccc1N2
(2)InChI: InChI=1/C7H6N2O/c10-7-8-5-3-1-2-4-6(5)9-7/h1-4H,(H2,8,9,10)
(3)InChIKey: SILNNFMWIMZVEQ-UHFFFAOYAP
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 620mg/kg (620mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 8, Pg. 42, 1958. |
Related Products
- 2-Hydroxybenzimidazole
- 61516-73-2
- 61516-78-7
- 615-18-9
- 615-20-3
- 615-21-4
- 6152-23-4
- 615-22-5
- 61522-53-0
- 61524-51-4
- 615258-01-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View