-
Name
2-Picolylamine
- EINECS 223-090-5
- CAS No. 3731-51-9
- Article Data55
- CAS DataBase
- Density 1.049
- Solubility soluble in water
- Melting Point 20 °C
- Formula C6H8N2
- Boiling Point 203 °C at 760 mmHg
- Molecular Weight 108.143
- Flash Point 90 °C
- Transport Information UN 2735 8/PG 2
- Appearance clear colorless to yellow or orange liquid
- Safety 26-36/37/39-45-25-36-27
- Risk Codes 36/37/38-34-37
-
Molecular Structure
-
Hazard Symbols
 C,
C, Xi
Xi
- Synonyms Pyridine,2-(aminomethyl)- (6CI,7CI,8CI);(2-Pyridyl)methanamine;1-(Pyridin-2-yl)methanamine;2-(Aminomethyl)pyridine;2-Picolinamine;2-Pyridinylmethylamine;NSC 59705;Pyridin-2-ylmethanamine;a-(Aminomethyl)pyridine;a-Picolylamine;
- PSA 38.91000
- LogP 1.24060
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water; dimethyl sulfoxide at 60℃; for 6h; High pressure; Green chemistry; | 99.9% |
| With 5%-palladium/activated carbon; ammonia; hydrogen In methanol at 30℃; under 7500.75 Torr; for 8h; Reagent/catalyst; Temperature; Pressure; Autoclave; | 95.11% |
| With sodium tetrahydroborate In water at 25℃; for 1h; Sonication; | 80% |
-
-
183016-40-2
[(Benzhydryl-amino)-pyridin-2-yl-methyl]-phosphonic acid diethyl ester

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 6h; Heating; | 72% |
| With hydrogenchloride; water for 6h; Hydrolysis; Heating; |
-
-
62451-88-1
amino-[2]pyridyl-acetic acid

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With ethanol; acetic acid; zinc |
-
-
36226-31-0
N-(2-pyridylmethyl)urea

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With nickel dichloride at 50 - 80.3℃; Thermodynamic data; Kinetics; Mechanism; ΔH(excit), ΔS(excit); |
-
-
7385-99-1
2-quinolinylhydrazone-(2-pyridinecarboxaldehyde)

-
A
-
100-70-9
2-Cyanopyridine

-
B
-
3731-51-9
(2-aminomethylpyridine)

-
C
-
580-22-3
2-aminoquinoline

| Conditions | Yield |
|---|---|
| In water Mechanism; electroreduction, pH 3.0, phosphate buffers, pyrolytic graphite electrode; |

-
-
76809-35-3
2,2-dimethyl-3-(2-pyridylmethyl)-4-oxo-4H-1,3-benzoxazine

-
A
-
3731-51-9
(2-aminomethylpyridine)

-
B
-
69-72-7
salicylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 3h; Heating; | A 0.24 g B 0.44 g |
-
-
80500-19-2
Diphenyl-[(pyridin-2-ylmethyl)-amino]-methanol

-
A
-
3731-51-9
(2-aminomethylpyridine)

-
B
-
119-61-9
benzophenone

| Conditions | Yield |
|---|---|
| With water In acetonitrile at 30℃; Rate constant; |
-
-
29668-43-7
2,3-dihydro-benzo[1,4]dioxine-5-carbaldehyde

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With lithium borohydride; Rink amine resin; water; trifluoroacetic acid; trimethyl orthoformate solid phase synthesis; 1) RT, 2) THF, 70 deg C, 5 h, 3) CH2Cl2, RT, 5 h; Yield given. Multistep reaction; |
-
-
15192-56-0
2-(diphenylmethyliminomethyl)pyridine

-
A
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene / 2 h / Heating 2: HCl; water / 6 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene / 1 h / Heating 2: 100 - 110 °C 3: 72 percent / aq. HCl / 6 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: ammonia / tetrahydrofuran / 24 h / 20 °C 2: sodium tetrahydroborate; methanol / 20 °C View Scheme |
-
-
15192-56-0
2-(diphenylmethyliminomethyl)pyridine

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 - 110 °C 2: 72 percent / aq. HCl / 6 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With carbonylchlorohydrido(4,5-bis((diisopropylphosphino)methyl)acridine)ruthenium(II); ammonia In toluene under 5700.38 Torr; for 30h; Inert atmosphere; Reflux; | 96 %Chromat. |
-
-
586-98-1
2-Hydroxymethylpyridine

-
A
-
3731-51-9
(2-aminomethylpyridine)

-
B
-
119715-60-5
N-(2-pyridylmethyl)-2-pyridylmethanimine

| Conditions | Yield |
|---|---|
| With ammonia; carbonylchlorohydrido(4,5-bis((diisopropylphosphino)methyl)acridine)ruthenium(II) In toluene under 5700.38 Torr; for 30h; Product distribution / selectivity; Reflux; |
-
-
1262670-63-2
2-(1-(triphenylmethylamino)methyl)pyridine

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate; water; acetic acid In dichloromethane at 20℃; for 0.0833333h; Inert atmosphere; |
-
-
609770-35-6
2-azidomethylpyridine

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen at 20℃; for 3h; |
-
-
609770-35-6
2-azidomethylpyridine

-
A
-
3731-51-9
(2-aminomethylpyridine)

-
-
132532-04-8, 137769-34-7
(R)-(+)-2-(diphenylphosphinyl)-2'-[(trifluoromethanesulfonyl)oxy]-1,1'-binaphthyl

| Conditions | Yield |
|---|---|
| In toluene at 115℃; for 20h; Staudinger Azide Reduction; Schlenk technique; Inert atmosphere; |

-
-
58481-18-8
N-(pyridin-2-ylmethyl)acetamide

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With [RuCl2(2-(diphenylphosphino)-N-((6-((diphenylphosphino)methyl)pyridin-2-yl)methyl)ethan-1-amine)]; potassium tert-butylate; hydrogen In tetrahydrofuran at 100℃; under 37503.8 Torr; for 20h; Catalytic behavior; Autoclave; chemoselective reaction; | 95 %Chromat. |
-
-
7166-34-9, 115663-05-3
pyridine-2-carbaldehyde imine

-
-
3731-51-9
(2-aminomethylpyridine)

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 20℃; | |
| Stage #1: pyridine-2-carbaldehyde imine With C66H114B2Ge2Li2N2O4 In hexane; toluene for 0.5h; Inert atmosphere; Schlenk technique; Stage #2: With water In hexane; toluene Inert atmosphere; Schlenk technique; |
-
-
100-70-9
2-Cyanopyridine

-
A
-
3731-51-9
(2-aminomethylpyridine)

-
B
-
1452-77-3
pyridine-2-carboxylic acid amide

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water; isopropyl alcohol at 80℃; under 15001.5 Torr; for 24h; Autoclave; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
50-00-0
formaldehyd

-
-
41661-56-7
1-(2-pyridinylmethyl)-piperidin-4-one

| Conditions | Yield |
|---|---|
| With methanol; acetic acid | 100% |

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
116953-37-8
2-O-methyl-1-O-(octadecylcarbamoyl)-3-O-(phenoxycarbonyl)glycerol

-
-
116953-38-9
2-O-methyl-3-O--1-O-(octadecylcarbamoyl)glycerol

| Conditions | Yield |
|---|---|
| at 90℃; for 1h; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
109-94-4
formic acid ethyl ester

-
-
56625-03-7
N-((pyridin-2-yl)methyl)formamide

| Conditions | Yield |
|---|---|
| for 1.5h; Reflux; | 100% |
| at 25℃; for 8h; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
154198-11-5
4-Bromo-2-formyl-6-(morpholin-4-ylmethyl)phenol

| Conditions | Yield |
|---|---|
| In toluene for 4h; Heating; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
37942-07-7
3,5-di-tert-butyl-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| In methanol for 2h; Heating; | 100% |
| In methanol for 1h; Ambient temperature; | |
| In methanol at 50℃; for 4h; | |
| In methanol Reflux; |
-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
119715-60-5
(E)-1-(pyridine-2-yl)-N-(pyridine-2-ylmethylene)methaneamine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 3h; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; for 3h; | 100% |
| In dichloromethane at 20℃; for 12h; Molecular sieve; | 98% |
| In ethanol |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
6404-29-1
6-(tert-butoxycarbonylamino)hexanoic acid

-
-
346693-19-4
1-(N-pyrid-2-ylmethyl)-6-(tert-butoxycarbonylamino)hexanamide

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 18h; | 100% |
| With triethylamine; dicyclohexyl-carbodiimide In ethyl acetate at 20℃; for 48h; | 83% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
66441-23-4, 71283-80-2
fenoxaprop-p-ethyl

-
A
-
71301-98-9
(R)-2-(4-hydroxyphenoxy)propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 20℃; | A n/a B 100% |

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
14562-10-8
2-hydroxy-3-phenylbenzaldehyde

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
156660-22-9
2,2'-dihydroxybiphenyl-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Aminomethyl)pyridine; benzaldehyde In methanol at 20℃; for 2h; Stage #2: With methanol; sodium tetrahydroborate at 20℃; for 1h; Cooling with ice; | 100% |
| Stage #1: 2-(Aminomethyl)pyridine; benzaldehyde With acetic acid In methanol at 20℃; for 1h; Stage #2: With sodium tetrahydroborate In methanol at 0℃; for 0.5h; | 80% |
| With sodium tetrahydroborate In ethanol for 12h; Heating; | 75% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
5241-64-5
Boc-D-Trp-OH

-
-
956489-59-1
(R)-tert-butyl 3-(1H-indol-3-yl)-1-oxo-1-(pyridin-2-ylmethylamino)propan-2-ylcarbamate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In dichloromethane at 20℃; for 1h; | 100% |
| With 4-methyl-morpholine In dichloromethane at 20℃; for 1h; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
13214-66-9
4-phenyl-1-butylamine

-
-
6515-58-8
3-bromomethylbenzoic acid

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: C17H17F3O3; di(succinimido) carbonate With triethylamine In acetonitrile at 20℃; for 1h; Stage #2: 2-(Aminomethyl)pyridine In acetonitrile at 20℃; for 0.25h; Further stages.; | 100% |

-
-
154492-12-3
[(1R,2R,4S)-4-[(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0^4,9]hexatriaconta-16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl] (4-nitrophenyl) carbonate

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
156598-70-8
[(1R,2R,4S)-4-[(2R)-2-[(1R,9S,12SR,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32SR,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0^4,9]hexatriaconta-16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl] N-(2-pyridylmethyl)carbamate

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at -20℃; for 1.5h; Inert atmosphere; | 100% |
| In dichloromethane at -10 - 0℃; for 3h; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
814-49-3
diethyl chlorophosphate

-
-
149543-47-5
pyridin-2-ylmethyl-phosphoramidic acid diethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 21h; Heating / reflux; | 100% |
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; | 85% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
623-27-8
terephthalaldehyde,

-
-
297771-20-1
N,N′-(1,4-phenylenebis(methylene))bis(1-(pyridin-2-yl)methanamine)

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Aminomethyl)pyridine; terephthalaldehyde, In benzene Heating / reflux; Stage #2: With hydrogen; palladium 10% on activated carbon In methanol under 1551.49 Torr; for 20h; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
298181-91-6
4-[[N-(tert-butoxycarbonyl)-N-(2-pyridinylmethyl)amino]methyl]benzylaldehyde

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
190728-25-7
4-((6,7-dimethoxyquinolin-4-yl)oxy)aniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
144-55-8
sodium hydrogencarbonate

| Conditions | Yield |
|---|---|
| With triethylamine In toluene | 100% |

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
70197-13-6
methyltrioxorhenium(VII)

| Conditions | Yield |
|---|---|
| In toluene decanted, washed with toluene, dried in vac.; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Aminomethyl)pyridine With copper(II) acetate monohydrate In ethanol for 24h; in air; Stage #2: With edetate disodium In chloroform; water | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
105-34-0
methyl 2-cyanoacetate

-
-
84951-58-6
2-cyano-N-(pyridin-2-ylmethyl)acetamide

| Conditions | Yield |
|---|---|
| at 0 - 20℃; Neat (no solvent); | 100% |
| In neat (no solvent) at 20℃; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
372948-82-8
2-pivaloylaminopyridine-6-carboxaldehyde

-
-
831195-44-9
N-(6-(((pyridin-2-ylmethyl)amino)methyl)pyridin-2-yl)pivalamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Aminomethyl)pyridine; 2-pivaloylaminopyridine-6-carboxaldehyde In methanol at 20℃; for 2.5h; Stage #2: With sodium tetrahydroborate at 0℃; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile | 100% |
| In acetonitrile at 25℃; | |
| In acetonitrile at 25℃; |


| Conditions | Yield |
|---|---|
| at 20℃; Inert atmosphere; Schlenk technique; Glovebox; | 100% |
| In methanol at 20℃; |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
219946-62-0
D-arabinono-1,4-lactone 5-(dihydrogenophosphate)

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide at 20℃; for 1.5h; Inert atmosphere; | 100% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
119715-60-5
N-(2-pyridylmethyl)-2-pyridylmethanimine

| Conditions | Yield |
|---|---|
| With NC-800; air In water; dimethyl sulfoxide at 120℃; for 8h; Green chemistry; | 99.9% |
| With porphyrin based sp2 carbon conjugated covalent organic framework; air In chlorobenzene; acetonitrile at 25℃; for 0.75h; Irradiation; | 96% |
| With oxygen at 100℃; under 3800.26 Torr; for 2h; Autoclave; Neat (no solvent); | 90% |
-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
3731-51-9
(2-aminomethylpyridine)

-
-
1539-42-0
bis[(2-pyridyl)methyl]amine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol | 99.6% |
| Stage #1: pyridine-2-carbaldehyde; 2-(Aminomethyl)pyridine In methanol at 20℃; for 10h; Stage #2: With methanol; sodium tetrahydroborate at 20℃; | 95% |
| Stage #1: pyridine-2-carbaldehyde; 2-(Aminomethyl)pyridine In methanol at 20℃; for 1h; Stage #2: With methanol; sodium tetrahydroborate at 0 - 20℃; | 95% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
134807-28-6
2-[N-(tert-butoxycarbonyl)aminomethyl]pyridine

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; | 99% |
| In dichloromethane Ambient temperature; | 98% |
| With 8Na(1+)*12C4H10NO(1-)*2HO(1-)*2Nd(3+) In neat (no solvent) at 20℃; for 0.0333333h; Inert atmosphere; Schlenk technique; Green chemistry; | 98% |
2-Picolylamine Chemical Properties
The Molecular Weight of 2-AMINOMETHYLPYRIDINE(3731-51-9) : 108.14
Molecular Structure:
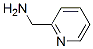
EINECS: 223-090-5
Melting point: 20°C
Boiling Point: 203 °C at 760 mmHg
Flash Point: 90 °C
Index of Refraction: 1.551
Molar Refractivity: 32.79 cm3
Molar Volume: 102.6 cm3
Polarizability: 13 10-24 cm3
Surface Tension: 45.9 dyne/cm
Density: 1.053 g/cm3
Enthalpy of Vaporization: 43.92 kJ/mol
Vapour Pressure: 0.284 mmHg at 25°C
Water Solubility: SOLUBLE
BRN: 108054
Synonyms:2-(Aminobenzyl)pyridine;2-pyridinemethanamine;beta-Picolylamine;2-aminomethyl-pyridin;2-Picolinamine;2-Pyridinemethylamine;2-Pyridinylmethanamine; (2-Pyridylmethyl)amine;
2-Picolylamine Toxicity Data With Reference
| 1. | ivn-mus LD50:340 mg/kg | APFRAD Annales Pharmaceutiques Francaises. 26 (1968),345. | ||
| 2. | orl-qal LD50:750 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. | ||
| 3. | orl-bwd LD50:562 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. | ||
| 4. | ivn-mus LD50:340 mg/kg | APFRAD Annales Pharmaceutiques Francaises. 26 (1968),345. |
2-Picolylamine Consensus Reports
2-Picolylamine Safety Profile
The Hazard Codes of2-AMINOMETHYLPYRIDINE(3731-51-9):
 C,
C,  Xi
Xi Hazard Note : Corrosive
HazardClass: 8
The Risk Statements information of 2-AMINOMETHYLPYRIDINE(3731-51-9):
34: Causes burns
37: Irritating to the respiratory system
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 2-AMINOMETHYLPYRIDINE(3731-51-9):
25: Avoid contact with eyes
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
27: Take off immediately all contaminated clothing
36: Wear suitable protective clothing
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN 2735 8/PG 2
WGK Germany: 3
RTECS: US1840000
PackingGroup: III
HS Code: 29333999
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View