-
Name
1-AMINO-3,3-DIETHOXYPROPANE
- EINECS
- CAS No. 41365-75-7
- Article Data13
- CAS DataBase
- Density 0.915 g/cm3
- Solubility
- Melting Point
- Formula C7H17NO2
- Boiling Point 200.6 °C at 760 mmHg
- Molecular Weight 147.217
- Flash Point 79.3 °C
- Transport Information
- Appearance clear colourless to yellow liquid
- Safety 26-27-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 3,3-Diethoxy-1-propanamine;3,3-Diethoxypropylamine;3-AMINOPROPIONALDEHYDE DIETHYL ACETAL;3,3-DIETHOXY-1-PROPYLAMINE;1-AMINO-3,3-DIETHOXYPROPANE;APEA;RARECHEM AL BW 0123;TIMTEC-BB SBB005781
- PSA 44.48000
- LogP 1.43460
Synthetic route
-
-
107833-73-8
3-nitropropanal diethyl acetal

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 7600.51 Torr; for 12h; | 98% |
-
-
2453-90-9
N-(3,3-diethoxypropyl)phthalimide

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With hydrazine In methanol for 2h; Heating; | 87% |
| With hydrazine In methanol; dichloromethane | 70% |
-
-
2032-34-0
3,3-bis(ethyloxy)propanenitrile

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 16h; Ambient temperature; | 49% |
| With lithium aluminium tetrahydride |
-
-
35573-93-4
3-chloro-1,1-diethoxy-propane

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With ethanol; ammonia | |
| With sodium azide; hydrogen; palladium on activated charcoal 1.) DMF, 2.) C2H5OH, 4 d; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: NaN3 / dimethylsulfoxide / 16 h / 50 °C 2: 8.2 g / H2 / 5 percent Pd/C / methanol / 2 h / 20 °C / 2585.74 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: 83 percent / dimethylformamide / 5 h / 145 °C 2: 87 percent / hydrazine / methanol / 2 h / Heating View Scheme |
-
-
35573-93-4
3-chloro-1,1-diethoxy-propane

-
A
-
41365-75-7
aminopropane diethyl acetal

-
B
-
854253-50-2
bis-(3,3-diethoxy-propyl)-amine

| Conditions | Yield |
|---|---|
| With ethanol; ammonia at 115 - 118℃; |
-
-
41365-74-6
3,3-diethoxypropionic amide

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether |

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With ammonia at 80℃; |
-
-
688317-69-3
3-azidopropanal diethyl acetal

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal; 5% Pd on active carbon In methanol at 20℃; under 2585.74 Torr; for 2h; | 8.2 g |
-
-
10601-80-6
ethyl 3,3-diethoxypropanoate

-
A
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NH3 2: LiAlH4 / diethyl ether View Scheme |
-
-
122-51-0
orthoformic acid triethyl ester

-
A
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Zn / benzene 2: aq. NH3 3: LiAlH4 / diethyl ether View Scheme |

-
-
41365-75-7
aminopropane diethyl acetal

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
153815-24-8
1-(tert-butyloxycarbonylamino)-3,3-diethoxypropane

| Conditions | Yield |
|---|---|
| In acetonitrile | 100% |
| In dichloromethane at 20℃; for 2h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 16.25h; | 94% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
17341-93-4
2,2,2-Trichloroethyl chloroformate

-
-
380635-36-9
3-(2,2,2-trichloroethoxycarbonylamino)propionaldehyde diethyl acetal

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 2.16667h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
922-67-8
propynoic acid methyl ester

| Conditions | Yield |
|---|---|
| 100% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
164532-58-5
N-(3,3-diethoxypropyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 10h; | 100% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 2h; | |
| With triethylamine In dichloromethane at 0 - 20℃; | |
| With triethylamine In dichloromethane at 0℃; for 0.5h; | |
| With triethylamine In dichloromethane at 20℃; for 1h; Inert atmosphere; |
-
-
956317-36-5
2-(2H-1,2,3-triazol-2-yl)-5-methylbenzoic acid

-
-
41365-75-7
aminopropane diethyl acetal

-
-
1410040-78-6
N-(3,3-diethoxypropyl)-5-methyl-2-(2H-1,2,3-triazol-2-yl)benzamide

| Conditions | Yield |
|---|---|
| With N-[(dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 15h; | 100% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 3h; | 1.6 g |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1610535-31-3
N-(4-chloro-6-prop-2-ynylamino-[1,3,5]triazin-2-yl)-O,N-dimethylhydroxylamine

-
-
1610534-70-7
N-[4-(3,3-diethoxypropylamino)-6-prop-2-ynylamino-[1,3,5]triazin-2-yl]-O,N-dimethylhydroxylamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 60℃; for 2h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1268621-10-8
5-{4-[4-({[(3-methylphenyl)amino]carbonyl}amino)phenoxy]pyridin-2-yl}-1H-pyrrole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-{4-[4-({[(3-methylphenyl)amino]carbonyl}amino)phenoxy]pyridin-2-yl}-1H-pyrrole-3-carboxylic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: aminopropane diethyl acetal In N,N-dimethyl-formamide at 20℃; for 0.166667h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide for 1h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide for 1h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 12h; | 100% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
54814-41-4, 54640-00-5, 54910-31-5
3-(3,4-dimethoxy-phenyl)-2-hydroxy-propionic acid methyl ester

-
-
170801-80-6
(R)-(+)-N-(3,3-Diethoxypropyl)-3-(3,4-dimethoxyphenyl)-2-hydroxypropionamide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 140℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 99% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1423-60-5
methyl ethynyl ketone

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 0.2h; | 99% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
876-08-4
p-(chloromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 99% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
2251-50-5
pentafluorobenzoylchloride

| Conditions | Yield |
|---|---|
| Stage #1: aminopropane diethyl acetal; pentafluorobenzoylchloride With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride; water In acetone for 1h; Inert atmosphere; Schlenk technique; | 99% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1613414-00-8
4-((3-((S)-phenyl(((((R)-quinuclidin-3-yl)oxy)carbonyl)amino)methyl)phenoxy)methyl)benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-((3-((S)-phenyl(((((R)-quinuclidin-3-yl)oxy)carbonyl)amino)methyl)phenoxy)methyl)benzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: aminopropane diethyl acetal at 20℃; for 18h; | 99% |
-
-
63810-78-6
4‑chloro‑5‑bromo‑2‑(methylthio)pyrimidine

-
-
41365-75-7
aminopropane diethyl acetal

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 3h; | 98% |
-
-
50-00-0
formaldehyd

-
-
41365-75-7
aminopropane diethyl acetal

-
-
14655-66-4
bis(4-methylphenyl)phosphinous acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid Kabachnik-Fields Reaction; Dean-Stark; Reflux; | 98% |

-
-
41365-75-7
aminopropane diethyl acetal

-
-
79878-15-2
2,4-di-(β,β,β-trifluoroethoxy)-5-nitropyridine

-
-
79878-17-4
4-(3',3'-diethoxypropylamino)-5-nitro-2-(β,β,β-trifluoroethoxy)-pyridine

| Conditions | Yield |
|---|---|
| In chloroform-d1 for 1h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
153381-08-9
Methyl (S)-(+)-3-(3,4 dimethoxyphenyl)-2-hydroxypropionate

-
-
153309-68-3
(S)-(-)-N-(3,3-Diethoxypropyl)-3-(3,4-dimethoxyphenyl)-2-hydroxypropionamide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 140℃; for 2h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
100-52-7
benzaldehyde

-
-
1099778-15-0
N-(3,3-diethoxypropyl)benzylamine hydrochloride

| Conditions | Yield |
|---|---|
| With chloroform; 10% Pd/C; hydrogen In ethanol at 20℃; under 1551.49 Torr; for 10h; chemoselective reaction; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
456-48-4
3-Fluorobenzaldehyde

-
-
1099778-13-8
3-fluoro-N-(3,3-diethoxypropyl)benzylamine hydrochloride

| Conditions | Yield |
|---|---|
| With chloroform; 10% Pd/C; hydrogen In ethanol at 20℃; under 1551.49 Torr; for 10h; chemoselective reaction; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
13049-86-0
diethyl 2-(ethoxycarbonylmethylene)malonate

-
-
1314160-55-8
C18H33NO8

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 18h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1268619-84-6
5-[4-(2-fluoro-5-{[(2-fluoro-5-methylphenyl)amino]carbonyl}phenoxy)pyridin-2-yl]-1H-pyrrole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-[4-(2-fluoro-5-{[(2-fluoro-5-methylphenyl)amino]carbonyl}phenoxy)pyridin-2-yl]-1H-pyrrole-3-carboxylic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: aminopropane diethyl acetal In N,N-dimethyl-formamide for 0.166667h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
1268619-99-3
5-(4-{4-fluoro-3-[(3-methyl-2-furoyl)amino]phenoxy}pyridin-2-yl)-1H-pyrrole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-(4-{4-fluoro-3-[(3-methyl-2-furoyl)amino]phenoxy}pyridin-2-yl)-1H-pyrrole-3-carboxylic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: aminopropane diethyl acetal In N,N-dimethyl-formamide for 0.166667h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
98-09-9
benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane; water at 0℃; for 2h; | 97% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
345-18-6
2-fluoro-5-chloronitrobenzene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 80℃; for 4.5h; | 97% |
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 80℃; for 4.5h; | 97% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 3h; | 96% |
-
-
41365-75-7
aminopropane diethyl acetal

-
-
83-01-2
diphenylcarbamic chloride

| Conditions | Yield |
|---|---|
| With triethylamine In benzene | 96% |
3,3-Diethoxypropylazanium Specification
The 3,3-Diethoxypropylazanium is an organic compound with the formula C7H17NO2. The IUPAC name of this chemical is 3,3-diethoxypropylazanium. With the CAS registry number 41365-75-7, it is also named as 1-Amino-3,3-diethoxypropane. Besides, it should be stored in a closed cool and dry place.
Physical properties about 3,3-Diethoxypropylazanium are: (1)ACD/LogP: 0.79; (2)ACD/LogD (pH 5.5): -2.14; (3)ACD/LogD (pH 7.4): -0.73; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1.92; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 7; (11)Polar Surface Area: 21.7 Å2; (12)Index of Refraction: 1.428; (13)Molar Refractivity: 41.46 cm3; (14)Molar Volume: 160.8 cm3; (15)Polarizability: 16.43×10-24cm3; (16)Surface Tension: 29.9 dyne/cm; (17)Density: 0.915 g/cm3; (18)Flash Point: 79.3 °C; (19)Enthalpy of Vaporization: 43.68 kJ/mol; (20)Boiling Point: 200.6 °C at 760 mmHg; (21)Vapour Pressure: 0.321 mmHg at 25°C.
Preparation of 3,3-Diethoxypropylazanium: this chemical can be prepared by 3-Phthalimido-1,1-diethoxy-propan. This reaction will need reagent hydrazine and solvent methanol. The reaction time is 2 hours by heating. The yield is about 87%.
.gif)
Uses of 3,3-Diethoxypropylazanium: it can be used to produce N,N'-bis-(3,3-diethoxy-propyl)-succinamide. It will need reagent Et3N and solvent benzene.
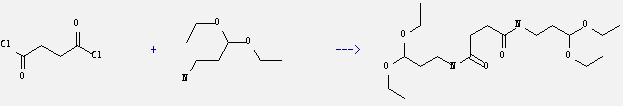
When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. When you are using it, wear suitable gloves and eye/face protection. Please take off immediately all contaminated clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O(CC)C(OCC)CCN
(2)InChI: InChI=1/C7H17NO2/c1-3-9-7(5-6-8)10-4-2/h7H,3-6,8H2,1-2H3
(3)InChIKey: PXXMSHBZYAOHBD-UHFFFAOYAQ
(4)Std. InChI: InChI=1S/C7H17NO2/c1-3-9-7(5-6-8)10-4-2/h7H,3-6,8H2,1-2H3
(5)Std. InChIKey: PXXMSHBZYAOHBD-UHFFFAOYSA-N
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 4136-95-2
- 4136-97-4
- 4137-10-4
- 41371-53-3
- 41372-02-5
- 41372-08-1
- 41372-10-5
- 41372-20-7
- 41372-22-9
- 41373-10-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View