-
Name
3-Methyl-1,5-pentanediol
- EINECS 224-709-1
- CAS No. 4457-71-0
- Article Data35
- CAS DataBase
- Density 0.974
- Solubility 1000g/L at 20℃
- Melting Point -60°C
- Formula C6H14 O2
- Boiling Point 228.6 °C at 760 mmHg
- Molecular Weight 118.176
- Flash Point 143 ºC
- Transport Information
- Appearance
- Safety Poison by intravenous route. Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, dry chemical, CO2. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOL, DENATURED; ALCOHOLS, C6-12; ALCOHOLS, C9-11; ALCOHOLS, C12-13, ETHOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED; ALCOHOLS, C12-16, ETHOXYLATED; ALCOHOLS, C14-15, ETHOXYLATED; ALCOHOLS, C16-18, ETHOXYLATED; ALCOHOLS, C8-10, ETHOXYLATED PROPOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED PROPOXYLATED; ALCOHOLS, N.O.S..
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,5-Dihydroxy-3-methylpentane;3-Methyl-1,5-pentanediol; Diol MPD; MPD; NSC 6496
- PSA 40.46000
- LogP 0.38730
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Heating; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Heating; | 68% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 2h; Reduction; | 44% |
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether for 6.5h; Heating; | 33% |
| With lithium aluminium tetrahydride In tetrahydrofuran |
-
-
18653-57-1
4-Methyl-3,4,5,6-tetrahydro-2H-pyran-2-ol

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With water; hydrogen; sodium hydroxide at 120℃; under 6000.6 Torr; for 6h; Reagent/catalyst; | 95% |
| With hydrogen; sodium hydroxide In water at 120℃; under 6000.6 Torr; for 7h; |

| Conditions | Yield |
|---|---|
| With borane-THF for 18h; Heating; | 75% |
| With lithium aluminium tetrahydride; diethyl ether | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 4h; | |
| Multi-step reaction with 2 steps 1: H2SO4 2: sodium; ethanol View Scheme |
-
-
19013-37-7
dimethyl 3-methylglutarate

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | 75% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 24h; Heating; |

| Conditions | Yield |
|---|---|
| With hydrogen; copper at 240℃; under 225018 Torr; for 5h; | 65% |
-
-
38113-08-5, 38320-49-9, 53608-95-0
3,4-dihydro-2-methoxy-4-methyl-2H-pyran

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With copper oxide-chromium oxide; water; hydrogen at 180℃; under 144160 Torr; | |
| With hydrogen; nickel; acetic acid at 150℃; under 66195.7 Torr; |
-
-
59677-19-9
6-methoxy-4-methyl-3,6-dihydro-2H-pyran

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With hydrogenchloride und Hydrieren des Reaktionsgemisches an Raney-Nickel in neutraler Loesung bei 125grad; |

| Conditions | Yield |
|---|---|
| With copper oxide-chromium oxide Hydrogenation; | |
| With lithium aluminium tetrahydride; diethyl ether | |
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 100℃; under 36775.4 Torr; Hydrogenation; |
-
-
40760-35-8
3-methylenepentane-1,5-diol

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; nickel at 100℃; under 257428 Torr; Hydrogenation; |
-
-
40065-27-8
1,5-diacetoxy-3-methylpentane

-
-
124-41-4
sodium methylate

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With methanol |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride |

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With copper chromite; hydrogen at 250℃; unter Druck; |

| Conditions | Yield |
|---|---|
| at 250℃; under 147102 - 220652 Torr; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: CuI / tetrahydrofuran / 20 °C 1.2: TMSCl / tetrahydrofuran / -78 - 20 °C 2.1: lithium aluminum hydride / tetrahydrofuran / 24 h / Heating View Scheme |
-
-
10138-44-0
2-ethoxy-4-methyl-3,4-dihydro-2H-pyran

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous sulfuric acid 2: Raney nickel; ethanol / 100 °C / 36775.4 Torr / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 200 °C 2: Raney nickel; dioxane / 100 °C / 257428 Torr / Hydrogenation View Scheme | |
| Multi-step reaction with 3 steps 1.1: A-36 ion exchange resin / 100 °C / 1200.12 Torr / Autoclave; Large scale 1.2: hollow molecular sieve nanotubes supported silicotungstic acid catalyst / 105 - 155 °C / 825.08 Torr / Large scale 2.1: dicarbonyl(acetylacotonato)rhodium(I); C72H100O6P2; hydrogen / 24 h / 125 °C / 75007.5 Torr / Flow reactor 3.1: hydrogen / 150 - 240 °C / 1200.12 Torr / Flow reactor View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Co2(CO)8 / 140 °C / 132391 Torr 2: LiAlH4 View Scheme |
-
-
117-84-0
Di-n-octyl phthalate

-
-
18653-57-1
4-Methyl-3,4,5,6-tetrahydro-2H-pyran-2-ol

-
A
-
1121-84-2
4-methyl-tetrahydro-pyran-2-one

-
B
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With manganese dioxide; copper chromite |

-
-
18653-57-1
4-Methyl-3,4,5,6-tetrahydro-2H-pyran-2-ol

-
A
-
1121-84-2
4-methyl-tetrahydro-pyran-2-one

-
B
-
4457-71-0
3-methylpentane-1,5-diol

-
C
-
956007-32-2
MPAE

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydrogen; nickel In water at 120℃; under 6600.66 Torr; for 5h; pH=8.4 - 10.9; Product distribution / selectivity; | |
| With hydrogen In water at 120℃; for 2h; Product distribution / selectivity; | |
| With hydrogen; cobalt In water at 120℃; for 5h; Product distribution / selectivity; |

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane |

| Conditions | Yield |
|---|---|
| With dicarbonylacetylacetonato rhodium (I); hydrogen; molybdenum hexacarbonyl In 1,4-dioxane at 200℃; under 90009 - 112511 Torr; for 2.33333h; Schlenk technique; Autoclave; | |
| With 5 wt% ruthenium/carbon; hydrogen In water at 100℃; under 105011 Torr; for 6.3h; | |
| With ruthenium-carbon composite; hydrogen In water at 100℃; under 105011 Torr; for 16h; Catalytic behavior; Temperature; Pressure; |
-
-
73746-79-9, 80698-15-3, 107242-87-5, 10603-03-9, 823-22-3
α-methyl-δ-valerolactone

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With 5 wt% ruthenium/carbon; hydrogen In water at 100℃; under 105011 Torr; for 6.3h; |

| Conditions | Yield |
|---|---|
| With ruthenium-carbon composite; hydrogen In water at 100℃; under 105011 Torr; for 3.5h; Catalytic behavior; Time; |
-
-
150-97-0, 690-72-2, 17817-88-8, 32451-23-3
D,L-mevalonic acid

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid / 15 h / Reflux 2: hydrogen; ruthenium-carbon composite / water / 3.5 h / 100 °C / 105011 Torr View Scheme |
-
-
101153-84-8
3-methyl-5-oxopentyl acetate

-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With hydrogen at 150 - 240℃; under 1200.12 Torr; Flow reactor; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 135℃; for 41h; | 99.6% |
| With Amberlyst 15 | 91% |
| With toluene-4-sulfonic acid at 135 - 155℃; Dean-Stark; Large scale; | 3662 g |
| With toluene-4-sulfonic acid at 135 - 155℃; for 41h; Large scale; | |
| With toluene-4-sulfonic acid at 135 - 155℃; |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
61898-56-4
(S)-4-methyltetrahydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With horse liver alcohol dehydrogenase; oxygen; catalase; 7-(trifluoromethyl)-1,10-ethyleneisoalloxazinium chloride In aq. phosphate buffer at 20℃; for 13h; pH=8; Catalytic behavior; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | 97% |
| With 2,6-dimethylpyridine; sodium perchlorate In acetonitrile for 10h; Electrolysis; | 96.4% |
| With sodium perchlorate; (-)-sparteine In acetonitrile macroelectrolysis with 2,2,6,6-tetramethylpiperidine-1-yloxyl)-modified graphite felt electrodes; | 93.8% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
148054-23-3
3-methylpentane-1,5-diyl bis(4-methylbenzenesulfonate)

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In acetonitrile at 20℃; for 3h; | 97% |
| With triethylamine In dichloromethane at 20℃; for 18h; | 82.5% |
| With pyridine at 20℃; for 2h; | |
| With pyridine at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| With oxygen In toluene at 110℃; for 1h; | 96% |
| With hydrotalcite-supported copper nanoparticles In 1,3,5-trimethyl-benzene at 150℃; for 8h; Inert atmosphere; | 96% |
| With [RuCl2(PPh3)2(2-PyCH21,3,5-triaza-7-phosphadamantane)].Br; potassium hydroxide In water for 48h; Reagent/catalyst; Schlenk technique; Reflux; Inert atmosphere; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium hydrogencarbonate In toluene at 110℃; for 16h; Microwave irradiation; Inert atmosphere; Sealed tube; | 96% |
| With 1,4-dioxane; copper oxide-chromium oxide; hydrogen at 250℃; under 147102 - 294203 Torr; |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
51174-46-0
3-methyl-1,5-diiodopentane

| Conditions | Yield |
|---|---|
| With phosphorus; iodine at 45℃; for 42h; Cooling with ice bath; | 87% |
| With phosphorus; iodine | |
| Multi-step reaction with 2 steps 1: 73 percent / PBr3 / 20 h / -10 - 20 °C 2: 89 percent / NaI / acetone / 3 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium hydrogencarbonate In toluene at 110℃; for 16h; Microwave irradiation; Inert atmosphere; Sealed tube; | 86% |

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 0℃; for 1h; | 85% |
| With hydrogen bromide In octane; water at 147 - 148℃; for 12h; Dean-Stark; | 83% |
| With phosphorus tribromide at -10 - 20℃; for 20h; Substitution; | 73% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydroxide at 40℃; for 7h; | 85% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
295318-34-2
5-(tert-butyldimethylsilyl)oxy-3-methyl-1-pentanol

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpentane-1,5-diol With n-butyllithium In tetrahydrofuran; hexane at 20℃; Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran; hexane at 20℃; for 0.75h; Inert atmosphere; Stage #3: With water; ammonium chloride | 84% |
| With sodium hydride In tetrahydrofuran Substitution; Silylation; | 83% |
| Stage #1: 3-methylpentane-1,5-diol With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; for 0.5h; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran; hexane at 20℃; | 52% |
| Stage #1: 3-methylpentane-1,5-diol With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran at 0℃; Inert atmosphere; | 51% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1076691-71-8
5-hydroxy-3-methylpentyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 3.16667h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; potassium hydroxide; catacxium A In toluene at 115℃; for 24h; Time; Sealed tube; chemoselective reaction; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpentane-1,5-diol With sodium hydride In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: 1,1'-(2-nitroethene-1,1-diyl)dibenzene In tetrahydrofuran at 15℃; for 15h; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpentane-1,5-diol With sodium hydride In tetrahydrofuran for 0.5h; Inert atmosphere; Stage #2: propargyl bromide In tetrahydrofuran Inert atmosphere; | 80% |
-
-
4457-71-0
3-methylpentane-1,5-diol

| Conditions | Yield |
|---|---|
| With diphenylphosphoranyl azide; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 65℃; for 16h; | 76% |

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; potassium hydroxide In toluene at 115℃; for 24h; Inert atmosphere; Sealed tube; diastereoselective reaction; | 76% |
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; potassium hydroxide In toluene at 115℃; for 24h; Sealed tube; Inert atmosphere; diastereoselective reaction; | 76% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
302-79-4
all-trans-retinoic-acid

-
-
1354766-27-0
3-methyl-1,5-diretinylpentanediester

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpentane-1,5-diol; all-trans-retinoic-acid With dmap; dicyclohexyl-carbodiimide In toluene at 20℃; for 15h; Darkness; Inert atmosphere; Stage #2: With hydrogenchloride In water; toluene | 74.5% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 0℃; for 5h; | 70% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
84268-33-7
2-[2'-hydroxy-3'-tert-butyl-5'-(2''-methoxycarbonylethyl)phenyl]-2H-benzotriazole

| Conditions | Yield |
|---|---|
| With di(n-butyl)tin oxide at 170℃; for 20h; Dean-Stark; Inert atmosphere; | 65% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
A
-
498-21-5, 636-60-2
2-methylbutanedioic acid

-
B
-
626-51-7
3-methyl glutaric acid

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide In nitric acid at 60℃; Heating; | A 62% B 23% |
| With dinitrogen tetraoxide In nitric acid at 50℃; Heating; | A 45% B 33% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
61898-55-3
(4R)-4-methyltetrahydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With acetate buffer pH 5 In water at 30℃; for 48h; Gluconobacter roseus IAM 1841; | 57% |
| Multi-step reaction with 3 steps 2: Jones oxidation 3: 1.) CH2N2, 2.) aq. HCl, MeOH View Scheme |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 12h; | 55% |
-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
1005-56-7
phenylcarbonochloridothioate

-
-
152429-69-1
O-(5-hydroxy-3-methylpentyl) O-phenyl thionocarbonate

| Conditions | Yield |
|---|---|
| With pyridine; dmap In acetonitrile at 0℃; for 5h; | 54% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; potassium hydroxide; catacxium A In toluene at 115℃; for 24h; Sealed tube; Overall yield = 77 percent; Overall yield = 135 mg; | A 51% B 26% |

-
-
4457-71-0
3-methylpentane-1,5-diol

-
-
814-68-6
acryloyl chloride

-
-
64194-22-5
3-methyl 1,5-pentanediol diacrylate

| Conditions | Yield |
|---|---|
| With triethylamine; 2-hydroxyresorcinol In benzene at 50℃; for 0.5h; | 50% |
3-Methyl-1,5-pentanediol Chemical Properties
Molecular Structure of 3-Methyl-1,5-pentanediol (4457-71-0):
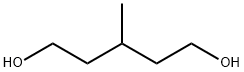
EINECS: 224-709-1
IUPAC Name: 3-Methylpentane-1,5-diol
Molecular Formula: C6H14O2
Molecular Weight: 118.174160 g/mol
XLogP3: 0.3
H-Bond Donor: 2
H-Bond Acceptor: 2
Canonical SMILES: CC(CCO)CCO
InChI: InChI=1S/C6H14O2/c1-6(2-4-7)3-5-8/h6-8H,2-5H2,1H3
InChIKey: SXFJDZNJHVPHPH-UHFFFAOYSA-N
Index of Refraction: 1.447
Molar Refractivity: 32.87 cm3
Molar Volume: 122.9 cm3
Surface Tension: 36.2 dyne/cm
Density: 0.961 g/cm3
Flash Point: 106.3 °C
Enthalpy of Vaporization: 54.08 kJ/mol
Boiling Point: 228.6 °C at 760 mmHg
Vapour Pressure: 0.0141 mmHg at 25 °C
Water Solubility: 2.617e+004 mg/L at 25 °C
Refractive Index: n20/D 1.454
BRN: 1697331
3-Methyl-1,5-pentanediol Toxicity Data With Reference
| 1. | ivn-mus LD50:320 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#01094 . |
3-Methyl-1,5-pentanediol Consensus Reports
Reported in EPA TSCA Inventory.
3-Methyl-1,5-pentanediol Safety Profile
Safety Information of 3-Methyl-1,5-pentanediol (4457-71-0):
Safety Statements: 23-24/25
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24/25: Avoid contact with skin and eyes
RIDADR: 1987
WGK Germany: 1
RTECS: SA0800000
Poison by intravenous route. Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, dry chemical, CO2. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOL, DENATURED; ALCOHOLS, C6-12; ALCOHOLS, C9-11; ALCOHOLS, C12-13, ETHOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED; ALCOHOLS, C12-16, ETHOXYLATED; ALCOHOLS, C14-15, ETHOXYLATED; ALCOHOLS, C16-18, ETHOXYLATED; ALCOHOLS, C8-10, ETHOXYLATED PROPOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED PROPOXYLATED; ALCOHOLS, N.O.S..
3-Methyl-1,5-pentanediol Specification
3-Methyl-1,5-pentanediol (4457-71-0) is known as 1,5-Dihydroxy-3-methylpentane ; 4-01-00-02571 (Beilstein Handbook Reference) ; AI3-28481 ; NSC 6496 ; 1,5-Pentanediol, 3-methyl- ; 3-Methylpentane-1,5-diol .
Related Products
- 3-Methyl-1-((2-methylcyclohexyl)carbonyl)piperidine
- 3-Methyl-1-((6-methyl-3-cyclohexen-1-yl)carbonyl)piperidine
- 3-Methyl-1-(2-phenylethyl)piperidin-4-one
- 3-Methyl-1-(3,4-dimethylphenyl)-2-pyrazolin-5-one
- 3-METHYL-1-(3'-SULFOAMIDOPHENYL)-5-PYRAZOLONE
- 3-Methyl-1-(4-methylphenyl)sulfonyl-urea
- 3-Methyl-1-(4-nitrophenyl)sulfonyl-urea
- 3-Methyl-1-(4-sulfophenyl)-2-pyrazolin-5-one
- 3-Methyl-1-(p-tolyl)-triazene
- 3-Methyl-1,2,4-thiadiazol-5-amine
- 4457-72-1
- 4458-15-5
- 4458-18-8
- 445-82-9
- 445-83-0
- 44601-24-3
- 446024-42-6
- 44603-18-1
- 446043-04-5
- 4460-60-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View