-
Name
3-Methylflavone-8-carboxylic acid
- EINECS 222-425-2
- CAS No. 3468-01-7
- Article Data5
- CAS DataBase
- Density 1.336 g/cm3
- Solubility
- Melting Point 234-236 °C
- Formula C17H12O4
- Boiling Point 485.1 °C at 760 mmHg
- Molecular Weight 280.28
- Flash Point 183.3 °C
- Transport Information
- Appearance White crystalline solid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 8-Carboxy-3-methylflavone;3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylicacid;
- PSA 67.51000
- LogP 3.46660
Synthetic route

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-benzoyloxy-3-propionylbenzoate With aluminum oxide at 165℃; for 4h; Stage #2: With sodium hydroxide In methanol; water at 75 - 80℃; for 1.5h; Temperature; | 88% |
-
-
103085-54-7
8-formyl-3-methyl-4-oxo-2-phenyl-4H-1-benzopyran

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In butanone at 90 - 95℃; for 44h; | 85% |
| With nitric acid In benzene Product distribution; Heating; various concentrations of HNO3.; | 85% |

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94 percent / NaBr, H2SO4 / acetonitrile; H2O / 2.83 h / 45 °C / electrolysis 2: 1) H2SO4; 2) electrolysis / 1) MeOH, 60 deg C, 40 min; 2) 60 deg C, 48 h. 3: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme | |
| Multi-step reaction with 4 steps 1: 94 percent / NaBr, H2SO4 / acetonitrile; H2O / 2.83 h / 45 °C / electrolysis 2: 94 percent / 10percent H2SO4 / tetrahydrofuran / 11 h / Ambient temperature 3: 91 percent / H2SO4 / methanol / 6 h / Ambient temperature; electrolysis 4: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme | |
| Multi-step reaction with 4 steps 1: 94 percent / NaBr, H2SO4 / acetonitrile; H2O / 2.83 h / 45 °C / electrolysis 2: 94 percent / 10percent H2SO4 / tetrahydrofuran / 11 h / Ambient temperature 3: 74 percent / H2SO4, electrolysis / methanol / 50 °C / other temperatures, other reagent, other solvent. 4: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme |
-
-
103085-50-3
8-(1,2-epoxypropyl)-3-methyl-2-phenyl-4H-1-benzopyran-4-one

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) H2SO4; 2) electrolysis / 1) MeOH, 60 deg C, 40 min; 2) 60 deg C, 48 h. 2: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme | |
| Multi-step reaction with 3 steps 1: 94 percent / 10percent H2SO4 / tetrahydrofuran / 11 h / Ambient temperature 2: 91 percent / H2SO4 / methanol / 6 h / Ambient temperature; electrolysis 3: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme | |
| Multi-step reaction with 3 steps 1: 94 percent / 10percent H2SO4 / tetrahydrofuran / 11 h / Ambient temperature 2: 74 percent / H2SO4, electrolysis / methanol / 50 °C / other temperatures, other reagent, other solvent. 3: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme |
-
-
103085-53-6
8-(1,2-dihydroxypropyl)-3-methyl-2-phenyl-4H-1-benzopyran-4-one

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / H2SO4 / methanol / 6 h / Ambient temperature; electrolysis 2: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme | |
| Multi-step reaction with 2 steps 1: 74 percent / H2SO4, electrolysis / methanol / 50 °C / other temperatures, other reagent, other solvent. 2: 85 percent / HNO3 / benzene / Heating; various concentrations of HNO3. View Scheme |

-
-
90101-87-4
Methyl 3-methylflavone-8-carboxylate

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| In methanol; water | 27.3 g (97.5%) |

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: flavoxate hydrochloride With sodium hydroxide for 3h; Reflux; Stage #2: With hydrogenchloride; water |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: bromine / dichloromethane / 4 h / 0 - 15 °C 1.2: 2.5 h / 60 - 65 °C 1.3: 1.5 h / 20 °C 2.1: sodium hydroxide / dichloromethane / 0 - 25 °C 3.1: aluminum oxide / 4 h / 165 °C 3.2: 1.5 h / 75 - 80 °C View Scheme |

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; 2,2-dimethylpropanoic anhydride; bis[2-(diphenylphosphino)phenyl] ether; sodium chloride In 1,4-dioxane at 150℃; for 10h; Heck Reaction; Schlenk technique; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 130℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; | 93% |
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane for 18h; Inert atmosphere; Schlenk technique; Heating; | 93% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane for 18h; Inert atmosphere; Schlenk technique; Heating; | 93% |
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 130℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; | 91% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
14717-29-4
dicyclohexylphosphine oxide

| Conditions | Yield |
|---|---|
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 130℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; | 83% |
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane for 18h; Inert atmosphere; Schlenk technique; Heating; | 83% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

| Conditions | Yield |
|---|---|
| With dmap; cesium fluoride; trifluoroacetic anhydride at 120℃; for 15h; Schlenk technique; Inert atmosphere; | 80% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
121-44-8
triethylamine

-
-
90101-88-5
N,N-diethyl-3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxamide

| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; palladium diacetate; 2,2-dimethylpropanoic anhydride In toluene at 120℃; for 15h; Inert atmosphere; | 75% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; palladium diacetate; 2,2-dimethylpropanoic anhydride In toluene at 150℃; for 15h; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylflavone-8-carboxylic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: 8-amino quinoline With triethylamine In dichloromethane at 0 - 45℃; for 12h; | 73% |

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; 2,2-dimethylpropanoic anhydride; palladium dichloride In 1,4-dioxane at 150℃; for 16h; Sonogashira Cross-Coupling; Inert atmosphere; Schlenk technique; | 70% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 115℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; | 68% |
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 115℃; for 18h; Inert atmosphere; Schlenk technique; | 68% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
172487-18-2
ethyl Phenylphosphinate

| Conditions | Yield |
|---|---|
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane at 130℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; | 67% |
| With di-tert-butyl dicarbonate; N,N-dimethyl-cyclohexanamine; 1,3-bis-(diphenylphosphino)propane; palladium diacetate In 1,4-dioxane for 18h; Inert atmosphere; Schlenk technique; Heating; | 67% |
-
-
14365-73-2
2,3,4-tri-O-acetyl-β-D-glucopyranosylamine uronic acid methyl ester

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; for 12h; | 49% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; isopropyl alcohol for 0.5h; Heating; | 32% |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 49 percent / HOBT; DMAP; DCC / tetrahydrofuran / 12 h / 20 °C 2: 84 percent / LiOH / methanol; tetrahydrofuran; H2O / 0 °C View Scheme |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
151860-17-2
(1α,5α,6α)-3-N-benzyl-6-amino-3-aza-bicyclo[3.1.0]hexane

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 4-methyl-morpholine; DMF (N,N-dimethyl-formamide); 1-hydorxy benzotriazole at 0 - 20℃; for 3.5h; |
-
-
134574-95-1
1-aminomethyl-3-benzyl-3-azabicyclo [3.1.0] hexane

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 4-methyl-morpholine; DMF (N,N-dimethyl-formamide); benzotriazol-1-ol at 0 - 20℃; for 3.5h; |

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
153240-51-8
8-{3-[2-(3,4-Dimethoxyphenyl)-N-methylethylamino]propoxycarbonyl}-3-methyl-4-oxo-2-phenyl-4H-1-benzopyran hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In N-methyl-acetamide; ethanol; water; isopropyl alcohol |
-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
21279-77-6
1-(2-methoxyphenyl)-4-(3-chloropropyl)piperazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N-methyl-acetamide; ethanol | |
| With potassium carbonate In N-methyl-acetamide; ethanol |
-
-
90101-87-4
Methyl 3-methylflavone-8-carboxylate

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
100859-97-0
piperidinoethyl chloride

| Conditions | Yield |
|---|---|
| With sodium In N-hydroxyethylpiperidine; benzene |

-
-
110-89-4
piperidine

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
556-52-5
oxiranyl-methanol

-
-
86433-56-9
oxiranemethyl 2-phenyl-3-methyl-4-oxo-4H-1-benzopyran-8-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetonitrile; benzene |

-
-
54151-70-1
1-(2-aminoisopropyl)-piperidine

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
541-41-3
chloroformic acid ethyl ester

-
-
92606-55-8
N-[2-(1-Piperidyl)propyl]-3-methylflavone-8-carboxamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran |


-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
51950-71-1
3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carbonyl chloride

-
-
103085-54-7
8-formyl-3-methyl-4-oxo-2-phenyl-4H-1-benzopyran

| Conditions | Yield |
|---|---|
| With thionyl chloride; palladium In 5,5-dimethyl-1,3-cyclohexadiene; water |

-
-
7154-73-6
1-(2-aminoethyl)pyrrolidine

-
-
3468-01-7
3-methylflavone-8-carboxylic acid

-
-
51950-71-1
3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carbonyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; thionyl chloride In benzene |

3-Methylflavone-8-carboxylic acid Specification
The 3-Methylflavone-8-carboxylic acid, with the CAS registry number 3468-01-7, is also known as 8-Carboxy-3-methylflavone. It belongs to the product categories of Pharmaceutical Raw Materials; Pharmaceutical Material and Intermeidates; Di-substituted Flavones; All Inhibitors; Inhibitors; Heterocycles; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals. Its EINECS registry number is 222-425-2. This chemical's molecular formula is C17H12O4 and molecular weight is 280.27. What's more, its IUPAC name is called 3-Methyl-4-oxo-2-phenylchromene-8-carboxylic acid. It is the main active metabolite of flavoxate hydrochloride in human.
Physical properties about 3-Methylflavone-8-carboxylic acid are: (1)ACD/LogP: 3.47; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.94; (4)ACD/LogD (pH 7.4): 0.33; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 5.42; (8)ACD/KOC (pH 7.4): 1.35; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 52.6 Å2; (13)Index of Refraction: 1.642; (14)Molar Refractivity: 75.84 cm3; (15)Molar Volume: 209.7 cm3; (16)Surface Tension: 55.4 dyne/cm; (17)Density: 1.335 g/cm3; (18)Flash Point: 183.3 °C; (19)Enthalpy of Vaporization: 79.05 kJ/mol; (20)Boiling Point: 485.1 °C at 760 mmHg; (21)Vapour Pressure: 3.2E-10 mmHg at 25 °C.
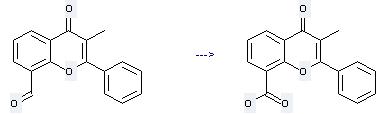
Preparation of 3-Methylflavone-8-carboxylic acid: this chemical can be prepared by 8-Formyl-3-methyl-2-phenyl-4H-1-benzopyran-4-one. This reaction needs reagent H2O2 and solvent butan-2-one at temperature of 90 - 95 °C. The reaction time is 44 hours. The yield is 85 %.
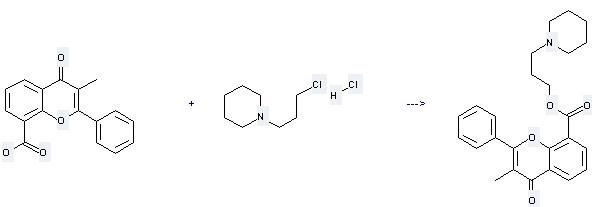
Uses of 3-Methylflavone-8-carboxylic acid: it is used to produce other chemicals. For example, it can react with 1-(3-Chloro-propyl)-piperidine; hydrochloride to get 3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylic acid 3-piperidin-1-yl-propyl ester. The reaction occurs with reagent KOH, solvents methanol, propan-2-ol and other condition of heating for 30 min. The yield is 32 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should wear suitable protective clothing. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(O)c3cccc1c3O/C(=C(\C1=O)C)c2ccccc2
(2) InChI: InChI=1S/C17H12O4/c1-10-14(18)12-8-5-9-13(17(19)20)16(12)21-15(10)11-6-3-2-4-7-11/h2-9H,1H3,(H,19,20)
(3) InChIKey: KMMBBZOSQNLLMN-UHFFFAOYSA-N
Related Products
- 3-Methylflavone-8-carboxylic acid
- 34680-81-4
- 3468-11-9
- 34681-29-3
- 3468-17-5
- 3468-18-6
- 34683-73-3
- 3468-63-1
- 34686-71-0
- 346-88-3
- 34688-71-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View