-
Name
4-Cyanopyridine
- EINECS 202-856-2
- CAS No. 100-48-1
- Article Data216
- CAS DataBase
- Density 1.12 g/cm3
- Solubility 3.2 g/100ml (16.4 °C) in water
- Melting Point 76-79 °C(lit.)
- Formula C6H4N2
- Boiling Point 196.3 °C at 760 mmHg
- Molecular Weight 104.111
- Flash Point 82.7 °C
- Transport Information 3276
- Appearance beige solid
- Safety 36/37-24/25
- Risk Codes 20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Isonicotinonitrile(6CI,8CI);4-Azabenzonitrile;4-Pyridinenitrile;4-Pyridylcyanide;4-Pyridylcarbonitrile;Isonicotinic acid nitrile;NSC 60681;g-Cyanopyridine;4-Pyridinecarbonitrile;
- PSA 36.68000
- LogP 0.95328
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia; oxygen; V*4Ti*4Sn*xO at 375℃; var. temp.; influence of water additions and heat-treatment temperature of catalyst; also 2-picoline; | 100% |
| With ammonia; oxygen; V*4Ti*4Sn*xO at 375 - 390℃; | 100% |
| 100% |
-
-
14906-59-3
4-cyanopyridine N-oxide

-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; tin(ll) chloride In diethyl ether for 0.5h; Ambient temperature; | 99% |
| With ammonia; lithium In tetrahydrofuran at -33℃; for 2h; other reagents: calcium, sodium, liq. ammonia; | 96% |
| With hexacarbonyl molybdenum In ethanol for 2h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With Palladium Nanoparticles with two shape-persistent covalent cages CC1' In N,N-dimethyl-formamide at 140℃; for 15h; Reagent/catalyst; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 3h; | 98% |
| With 1,1,3,3-tetraphenyl-2-oxa-1,3-phoshpinobenzene bis(trifluoromethanesulfonate); triethylamine In dichloromethane at 20℃; for 0.333333h; | 95% |
| With diethyl chlorophosphate In toluene for 4h; Beckmann rearrangement; Heating; | 94% |

-
-
1692-15-5
4-pyridylboronic acid

-
A
-
100-48-1
pyridine-4-carbonitrile

-
B
-
16937-03-4
4-nitro-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 8h; Catalytic behavior; Reflux; | A 98% B n/a |


| Conditions | Yield |
|---|---|
| With C18H14CuIN4 In acetonitrile at 20℃; for 24h; Inert atmosphere; Sealed tube; UV-irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium persulfate; sodium iodide; iron(II) chloride In 1,2-dichloro-ethane at 20 - 50℃; for 16h; | 96% |
| With ammonium acetate; phenyltrimethylammonium tribromide In dichloromethane at 20℃; for 21h; | 94% |
| With acetic acid; hydroxylamine-O-sulfonic acid In water at 50℃; for 6h; | 91% |

-
-
1692-15-5
4-pyridylboronic acid

-
A
-
100-48-1
pyridine-4-carbonitrile

-
B
-
6295-97-2
4-bromo-benzenesulfonic acid-(4-chloro-anilide)

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 10h; Catalytic behavior; Reflux; | A 96% B n/a |


| Conditions | Yield |
|---|---|
| With mesoporous silica SBA-15 supported Cu2O nanoparticles In N,N-dimethyl-formamide at 120℃; for 8h; Green chemistry; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; | 92% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 15h; Schlenk technique; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine In dichloromethane at 20℃; for 1h; | 94% |
| With methylene blue; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 25℃; Sealed tube; Irradiation; | 93% |
| With triphenyl bismuth (2+); dichloride; triethylamine In dichloromethane at 20℃; for 0.25h; | 85% |
-
-
15854-87-2
4-iodopyridine

-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium carbonate; potassium ferrocyanide In N,N-dimethyl-formamide at 120℃; for 5h; | 94% |
-
-
119986-58-2
N-(4-chlorophenyl)-N-cyano-4-methylbenzenesulfonamide

-
-
1692-15-5
4-pyridylboronic acid

-
A
-
100-48-1
pyridine-4-carbonitrile

-
B
-
2903-34-6
4-methyl-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 12h; Catalytic behavior; Reflux; | A 94% B n/a |

-
-
74037-41-5
(E)-4-((2,2-dimethylhydrazono)methyl)pyridine

-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid; methyltrioxorhenium(VII) In water; acetonitrile for 0.25h; | 92% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 240h; | 78% |
| With dihydrogen peroxide; 2-nitrobenzeneseleninic acid In methanol at 20℃; for 72h; | 65% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; aminosulfonic acid; urea for 0.133333h; Irradiation; | 90% |
| With ammonium acetate; acetic acid | |
| Multi-step reaction with 3 steps 1: concentrated sulfuric acid 2: alcohol; ammonia 3: phosphorus pentoxide / 25 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: ammonia / 0.5 h / 305 °C 2: 1 h / 330 °C View Scheme | |
| With aluminum oxide; vanadium; ammonia at 350℃; |
-
-
13958-98-0
3-bromo-4-cyanopyridine

-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With copper In N,N-dimethyl-formamide at 100 - 110℃; for 48h; | 90% |
-
-
15854-87-2
4-iodopyridine

-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; copper(II) acetate monohydrate In water at 20 - 140℃; Microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate; ammonium hydroxide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In acetonitrile at 20℃; for 5h; | 89% |
| Stage #1: pyridine-4-methanol With sodium azide; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; zinc trifluoromethanesulfonate In acetonitrile at 25℃; Irradiation; Stage #2: With trifluorormethanesulfonic acid In acetonitrile for 1h; | 78% |
| With ammonium hydroxide; oxygen In tert-Amyl alcohol at 130℃; under 3750.38 Torr; for 18h; | 85 %Chromat. |

| Conditions | Yield |
|---|---|
| With copper diacetate; Triphenylphosphine oxide; silver(l) oxide at 125℃; for 72h; | 88% |

| Conditions | Yield |
|---|---|
| With nicotinic acid In sulfolane at 190℃; for 0.25h; | 87% |

| Conditions | Yield |
|---|---|
| With bis(trimethylsilyl)amide yttrium(III) In toluene at 20℃; for 12h; Inert atmosphere; | A 87% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: pyridin-4-aldoxime With 2-chloro-1-methyl-pyridinium iodide In dichloromethane at 20℃; for 0.166667h; Stage #2: With triethylamine In dichloromethane at 20℃; for 1h; | 86% |

| Conditions | Yield |
|---|---|
| With vanadium oxide on hydrotalcite (V/HT) In 1,3,5-trimethyl-benzene for 48h; Reflux; | 83% |
| at 330℃; for 1h; Temperature; | 77% |
| With C36H38Cl6N6Pd3S2 In water; acetonitrile at 80℃; for 6h; Reagent/catalyst; | 67% |
-
-
694-59-7
pyridine N-oxide

-
-
7677-24-9
trimethylsilyl cyanide

-
A
-
100-70-9
2-Cyanopyridine

-
B
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 12h; Heating; | A 80% B 0.5 % Chromat. |

-
-
7757-39-3
pyridine-4-carbaldehyde phenylhydrazone

-
A
-
100-48-1
pyridine-4-carbonitrile

-
B
-
7684-30-2
N,N-dimethyl-N'-phenylcarbamimidic chloride

| Conditions | Yield |
|---|---|
| With dichloromethylenedimethyliminium chloride In 1,2-dichloro-ethane 1.) room temp., 1 h, 2.) reflux, 4 h; | A 80% B n/a |

| Conditions | Yield |
|---|---|
| With copper diacetate; acetonitrile for 1.5h; Reflux; | A 77% B 15 %Chromat. |

| Conditions | Yield |
|---|---|
| In diethyl ether; water for 5h; | 75% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite In ethanol at 0℃; for 0.25h; | 73% |
| With C68H64Cl2N6P2Ru2(4+)*2F6P(1-)*2Cl(1-); caesium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; Inert atmosphere; Green chemistry; | 76 %Chromat. |

| Conditions | Yield |
|---|---|
| In diethyl ether; water for 5h; | 72% |
-
-
696-54-8
pyridine-4-aldoxime

-
-
2942-58-7
diethyl cyanophosphonate

-
A
-
100-48-1
pyridine-4-carbonitrile

-
B
-
244016-79-3
C10H15N2O4P

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 20h; Reagent/catalyst; Inert atmosphere; | A 72% B 13% |

-
-
100-48-1
pyridine-4-carbonitrile

-
-
127-17-3
2-oxo-propionic acid

-
-
37398-49-5
2-acetylpyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With NH4S2O8; sulfuric acid; silver nitrate In dichloromethane; water at 40℃; for 2.5h; | 100% |

| Conditions | Yield |
|---|---|
| With NH4S2O8; sulfuric acid; silver nitrate In dichloromethane; water at 40℃; for 2.5h; | 100% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
98-89-5
Cyclohexanecarboxylic acid

-
-
83001-42-7
2,6-dicyclohexylisonicotinonitrile

| Conditions | Yield |
|---|---|
| With ammonium persulfate; sulfuric acid; silver nitrate In water at 50℃; for 2h; | 100% |
| With bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; for 12h; Inert atmosphere; Irradiation; Schlenk technique; | 74% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
14389-12-9
5-(4-pyridyl)tetrazole

| Conditions | Yield |
|---|---|
| With sodium azide; copper(II) sulfate In dimethyl sulfoxide at 140℃; for 1h; | 100% |
| Stage #1: pyridine-4-carbonitrile With sodium azide In N,N-dimethyl-formamide at 120℃; for 36h; Stage #2: With hydrogenchloride In water; ethyl acetate for 0.0833333h; | 99% |
| With sodium azide In N,N-dimethyl-formamide at 120℃; for 24h; | 99.4% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; titanium(III) chloride In water at 0℃; Mechanism; in the absence and in the presence of complex forming agents; | A 100% B n/a |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
64-17-5
ethanol

-
-
92259-16-0, 108748-88-5
isonicotinimidic acid ethyl ester; dihydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at 0 - 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; dimethyl sulfoxide at 60℃; for 8h; High pressure; Green chemistry; | 99.9% |
| With C13H26B(1-)*K(1+) In tetrahydrofuran for 24h; Ambient temperature; | 65% |
| With sulfuric acid; hydrogen In water at 50℃; for 3h; | 59% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water; dimethyl sulfoxide at 60℃; for 6h; High pressure; Green chemistry; | 99.9% |
| With [Ru(H)(BH4)(CO)(PPh3)(3-(di-tert-butylphosphino)-N-((1-methyl-1H-imidazol-2 yl)methyl)propylamine)]; hydrogen In isopropyl alcohol at 50℃; for 3h; Inert atmosphere; Autoclave; | 95% |
| With palladium 10% on activated carbon; ammonia; hydrogen In methanol at 20℃; under 3750.38 Torr; for 10h; Reagent/catalyst; Temperature; Pressure; Autoclave; | 93.9% |

| Conditions | Yield |
|---|---|
| With cobalt(II,III) oxide; water at 140℃; for 9h; | 99.8% |
| With water; potassium carbonate at 150℃; for 0.25h; Microwave irradiation; | 99% |
| With manganese(IV) oxide; water In isopropyl alcohol at 60℃; under 5171.62 Torr; for 0.25h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-4-carbonitrile With hydrogenchloride; acrylic acid at 90℃; for 1.5h; Inert atmosphere; Stage #2: dimethyl amine at 70℃; for 2.5h; Inert atmosphere; Stage #3: With sodium hydroxide at 70 - 90℃; for 3.5h; Temperature; Reagent/catalyst; Inert atmosphere; | 99.4% |
| Stage #1: pyridine-4-carbonitrile With hydrogenchloride; acrylic acid In water at 70℃; for 4h; Inert atmosphere; Stage #2: dimethyl amine In water at 50℃; for 3h; Temperature; Inert atmosphere; | 99% |
| Stage #1: pyridine-4-carbonitrile With hydrogenchloride; hydroquinone; acrylic acid In water at 90℃; for 6h; Stage #2: dimethyl amine In water for 3h; Temperature; Reflux; | 98.6% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
60-23-1
Cysteamine

-
-
106735-89-1
2-(pyridin-4-yl)-4,5-dihydrothiazole

| Conditions | Yield |
|---|---|
| With tribromomelamine at 100℃; for 0.05h; Neat (no solvent); | 99% |
| With 1-butyl-3-methylimidazolium tribromide at 100℃; for 0.133333h; | 98% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 110℃; for 0.05h; chemoselective reaction; | 96% |
| In ethanol Heating; |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
19962-42-6
(CH3)3SnCH2C6H4-p-CH3

-
-
79-22-1
methyl chloroformate

-
-
105621-37-2
4-Cyano-4-(4-methyl-benzyl)-4H-pyridine-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane 0 gradC for 2 h. then room temp. overnight; | 99% |

-
-
100-48-1
pyridine-4-carbonitrile

-
-
51755-57-8
(4-Methoxybenzyl)trimethylstannane

-
-
79-22-1
methyl chloroformate

-
-
105621-39-4
4-Cyano-4-(4-methoxy-benzyl)-4H-pyridine-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane 0 gradC for 2 h. then room temp. overnight; | 99% |


| Conditions | Yield |
|---|---|
| With sodium In tetrahydrofuran at 0 - 10℃; | 99% |
| With lithium In 5,5-dimethyl-1,3-cyclohexadiene Reagent/catalyst; Inert atmosphere; Reflux; | 96% |
| With sodium In xylene for 3h; Product distribution; Mechanism; Heating; other ketones and cyanopyridine; var. metals; var. sovents, temperatures and reaction time; | 70% |
| With sodium In xylene for 3h; Heating; | 70% |
| Stage #1: benzophenone With sodium In 5,5-dimethyl-1,3-cyclohexadiene at 105 - 130℃; for 0.333333h; Inert atmosphere; Stage #2: pyridine-4-carbonitrile In 5,5-dimethyl-1,3-cyclohexadiene at 125 - 130℃; for 2h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With fac-tris(2-phenylpyridinato-N,C2')iridium(III); N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 20℃; for 12h; Irradiation; Inert atmosphere; Sealed tube; | 99% |
| With sodium In tetrahydrofuran at 0 - 10℃; | 91% |
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammonium tetrafluoroborate In N,N-dimethyl-formamide at 20℃; for 6h; Sealed tube; Electrochemical reaction; | 85% |
| With pentanal; tetrabutylammonium acetate In dimethyl sulfoxide at 50℃; for 6h; Electrochemical reaction; | 57% |
| With bis(pinacolato)diborane In tert-butyl methyl ether at 90℃; for 24h; Temperature; Time; Inert atmosphere; Sealed tube; | 54% |
-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| In methanol N2; Co comp. dissolved under reflux, to a soln. added an excess of ligand, refluxed for 25 min, a soln. of NH4PF6 added; ppt. filtered, washed copiously (diethyl ether), dried (vac.); elem. anal.; | 99% |

-
-
100-48-1
pyridine-4-carbonitrile

-
-
359764-25-3
(COD)Pd(CH2CMe2C6H4)

-
-
359764-39-9
(CNC5H4N)2PdCH2C(CH3)2C6H4

| Conditions | Yield |
|---|---|
| In diethyl ether to a cooled (-30°C) suspn. of complex in diethyl ether was added a soln. of 4-cyanopyridine in diethyl ether, the mixt. was warmed to room temp., stirred at room temp. for 1 h (N2); evapd. to dryness, the solid was washed with petroleum ether and dried, recrystd. from CH2Cl2; elem. anal.; | 99% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
221314-94-9
Pd(P(CH3)3)2(CH2C(CH3)2C6H4)

-
-
359764-41-3
(P(CH3)3)(C4H9NC)PdCH2C(CH3)2C6H4

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: P(CH3)3; t-butylisocyanide in diethyl ether was added to a soln. of complex in diethyl ether at -30°C, the mixt. was stirred at room temp. for 1 h(N2); evapd. under reduced pressure, the residue was extd. with diethyl ether,partially concd., cooled to -30°C; | 99% |
-
-
100-48-1
pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| In methanol excess of N-compd. was added to hot MeOH soln. of Cu-complex, reflux for25 min, concd. MeOH soln. of NH4PF6 was added; ppt. was collected, washed with copious amt. of Et2O, dried in vac., elem. anal.; | 99% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
403-29-2
2-bromo-4'-fluoroacetophenone

-
-
1186423-28-8
4-cyano-1-(2-(4-fluorophenyl)-2-oxoethyl)pyridin-1-ium bromide

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 5h; Inert atmosphere; | 99% |
| In methanol at 20℃; | 98% |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
17013-01-3
fumaric acid disodium salt

| Conditions | Yield |
|---|---|
| In water stirring of Co(NO3)2*6H2O, Na2C4H2O4 and 4-cyanopyridine in water for 4 h; pptn., filtration, washing with H2O and drying in vac. desiccator over CaCl2; elem. anal.; | 99% |

-
-
100-48-1
pyridine-4-carbonitrile

-
-
1369917-99-6
3-fluoro-2,N,N-trimethylbenzamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluoro-2,N,N-trimethylbenzamide With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: pyridine-4-carbonitrile In tetrahydrofuran; hexane at -78 - 20℃; for 17h; | 99% |

| Conditions | Yield |
|---|---|
| With tris(bipyridine)ruthenium(II) dichloride hexahydrate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; ammonia; trifluoroacetic acid In acetonitrile at 60℃; for 15h; Sealed tube; Inert atmosphere; Irradiation; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium chloride In dimethyl sulfoxide at 20℃; for 10.72h; Glovebox; Inert atmosphere; Electrochemical reaction; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at 0℃; | 98.4% |
| With hydrogenchloride | |
| Stage #1: pyridine-4-carbonitrile; ethanol With hydrogenchloride Stage #2: With sodium hydroxide | |
| Stage #1: pyridine-4-carbonitrile; ethanol With hydrogenchloride In diethyl ether at 0 - 20℃; Stage #2: With triethylamine In ethanol for 18h; |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
109-73-9
N-butylamine

-
-
54433-75-9
N-(pyridine-4-ylmethylene)butan-1-amine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen In hexane at 80℃; under 750.075 Torr; for 24h; Autoclave; Green chemistry; | 98.1% |

| Conditions | Yield |
|---|---|
| With diammonium sulfide; 1,6-bis(3-methylimidazolium-1-yl)hexane dichloride at 70℃; for 0.0666667h; | 98% |
| With ammonium hydroxide; tetraphosphorus decasulfide In water at 40 - 50℃; for 0.5h; Reagent/catalyst; Time; | 98% |
| With boron trifluoride diethyl etherate; tiolacetic acid In 1,2-dichloro-ethane Ambient temperature; | 94% |
4-Cyanopyridine Specification
The 4-Cyanopyridine, with the CAS registry number 100-48-1, is also known as Isonicotinic acid nitrile. It belongs to the product category of Pyridines Derivates. Its EINECS registry number is 202-856-2. This chemical's molecular formula is C6H4N2 and molecular weight is 104.11. Its IUPAC name is called pyridine-4-carbonitrile. The product should be sealed and stored in cool and well-ventilated place. What's more, it should be protected from strong oxides. 4-Cyanopyridine is mainly used as the pharmaceutical, pesticide intermediates products.
Physical properties of 4-Cyanopyridine: (1)ACD/LogP: 0.41; (2)ACD/LogD (pH 5.5): 0.4; (3)ACD/LogD (pH 7.4): 0.4; (4)ACD/BCF (pH 5.5): 1.2; (5)ACD/BCF (pH 7.4): 1.2; (6)ACD/KOC (pH 5.5): 39.55; (7)ACD/KOC (pH 7.4): 39.56; (8)#H bond acceptors: 2; (9)Index of Refraction: 1.539; (10)Molar Refractivity: 29.11 cm3; (11)Molar Volume: 92.8 cm3; (12)Surface Tension: 51.4 dyne/cm; (13)Density: 1.12 g/cm3; (14)Flash Point: 82.7 °C; (15)Enthalpy of Vaporization: 43.25 kJ/mol; (16)Boiling Point: 196.3 °C at 760 mmHg; (17)Vapour Pressure: 0.401 mmHg at 25°C.
Preparation of 4-Cyanopyridine: this chemical can be prepared by 4-methyl-pyridine. This reaction is a kind of Oxidation. It will need reagents O2, ammonia and water. The yield is about 95%.

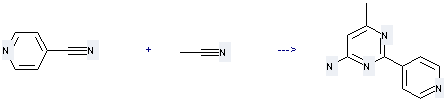
Uses of 4-Cyanopyridine: it can be used to produce 6-methyl-2-pyridin-4-yl-pyrimidin-4-ylamine with acetonitrile by heating. This reaction will need reagent LDA and solvent tetrahydrofuran with reaction time of 5 hours. The yield is about 85%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause damage to health. It is harmful by inhalation, in contact with skin and if swallowed. Whenever you will contact it, please wear suitable protective clothing and gloves. Finally, you must avoid contacting it with skin and eyes.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1=CN=CC=C1C#N
(2)InChI: InChI=1S/C6H4N2/c7-5-6-1-3-8-4-2-6/h1-4H
(3)InChIKey: GPHQHTOMRSGBNZ-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View