-
Name
4-Pentylbenzoic acid
- EINECS 247-607-9
- CAS No. 26311-45-5
- Article Data17
- CAS DataBase
- Density 1.043 g/cm3
- Solubility insoluble in water
- Melting Point 85-90 °C
- Formula C12H16O2
- Boiling Point 315.3 °C at 760 mmHg
- Molecular Weight 192.258
- Flash Point 149.7 °C
- Transport Information
- Appearance white to light yellow crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzoicacid, p-pentyl- (8CI);4-Amylbenzoic acid;4-n-Pentylbenzoic acid;NSC 169024;p-Amylbenzoic acid;p-Pentylbenzoic acid;
- PSA 37.30000
- LogP 3.11750
Synthetic route

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In tetrahydrofuran -78 deg C, 10 min, 20 deg C, 3.5 h; | 87% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon In 1,3,5-trimethyl-benzene at 200℃; Diels-Alder reaction; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I); potassium methanolate In tetrahydrofuran at 70℃; for 24h; Schlenk technique; Sealed tube; | 71% |

| Conditions | Yield |
|---|---|
| With carbon disulfide; aluminium trichloride |
-
-
6853-57-2
4-pentylbenzaldehyde

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With jones reagent In acetone for 0.5h; Ambient temperature; |

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hypobromide |

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 10 mg |
-
-
538-68-1
pentylbenzene

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic acid / 80 °C 2: Jones reagent / acetone / 0.5 h / Ambient temperature View Scheme |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
81720-37-8
4-n-pentylbenzyl alcohol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 16h; | 99% |
| With lithium aluminium tetrahydride In tetrahydrofuran | 60% |
| With lithium aluminium tetrahydride |
-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water | 94% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; potassium iodide at 100℃; for 24h; Schlenk technique; | 93% |

| Conditions | Yield |
|---|---|
| With acetyl chloride at 0 - 23℃; Inert atmosphere; | 92% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
38289-29-1, 38792-89-1, 67589-84-8
4-trans-pentylcyclohexane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-pentylbenzoic acid With sodium hydroxide; hydrogen In water at 140 - 150℃; under 22502.3 - 30003 Torr; for 1h; Stage #2: With sodium hydroxide In water at 260 - 280℃; under 22502.3 - 30003 Torr; for 2h; Further stages.; | 88% |
| With hydrogen Multistep reaction; | |
| In trans-4-butylcyclohexane carboxylic acid | 80% (43 g) |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; | 86% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
115914-43-7
bis{1,9-(4-hydroxyphenyl)nonane}

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Steglich Esterification; | 83% |
-
-
881650-28-8
5-[4-(4-heptylbenzoyl)-phenyl]-2-(4-hydroxyphenyl)-1,3,4-oxadiazole

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; for 48h; | 80% |
-
-
1188382-13-9
(3S,3αS,6R,8S,9βS)-6,8-dihydroxy-3,6,9-trimethyl-3α,4,5,6,6α,7,8,9β-octahydroazuleno[4,5-β]furan-2(3H)-one

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (3S,3αS,6R,8S,9βS)-6,8-dihydroxy-3,6,9-trimethyl-3α,4,5,6,6α,7,8,9β-octahydroazuleno[4,5-β]furan-2(3H)-one; 4-pentylbenzoic acid With dmap In dichloromethane at 20℃; for 0.166667h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane | 80% |
-
-
881649-30-5
5-[4-(4-dodecyloxybenzoyl)-phenyl]-2-(4-hydroxyphenyl)-1,3,4-oxadiazole

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; for 48h; | 79% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
79-19-6
thiosemicarbazide

-
-
114751-76-7
5-(4-pentylphenyl)-1,3,4-thiadiazol-2-amine

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 0 - 70℃; for 4.5h; | 78% |
| With trichlorophosphate at 0℃; for 4.5h; Reflux; |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
123843-58-3
3-fluoro-4'-hydroxy-1,1'-biphenyl-4-carbonitrile

| Conditions | Yield |
|---|---|
| With 4-pyrrolidin-1-ylpyridine; dicyclohexyl-carbodiimide In dichloromethane Ambient temperature; | 77% |
-
-
26311-45-5
4-pentylbenzoic acid


| Conditions | Yield |
|---|---|
| Stage #1: 4-pentylbenzoic acid; (3αS,6R,8S,9βS)-8-hydroxy-6,9-dimethyl-3-methylene-2-oxo-2,3,3α,4,5,6,6α,7,8,9β-decahydroazuleno[4,5-b]furan-6-yl acetate With dmap In dichloromethane at 20℃; for 0.166667h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 77% |
-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 2h; Inert atmosphere; | 76% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
6882-74-2
4,5,6,7-tetrahydro-1H-imidazo<4,5-c>pyridine

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-pentylbenzoic acid With 1,1'-carbonyldiimidazole In DMF (N,N-dimethyl-formamide) at 20℃; for 2h; Stage #2: 4,5,6,7-tetrahydro-1H-imidazo<4,5-c>pyridine With triethylamine In DMF (N,N-dimethyl-formamide) at 20℃; Stage #3: oxalic acid In ethyl acetate at 20℃; | 73% |


| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 2h; | 72.4% |
-
-
55996-28-6
2-methoxy-6-methyl-4(3H)-pyrimidinone

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In tetrahydrofuran 1.) reflux, 2 h; 2.) RT, 18 h; | 71% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
1342883-62-8
4-[3-(4-hydroxyphenyl)-1,2,4-oxadiazol-5-yl]phenyl 4-hexyl-benzoate

-
-
1342883-87-7
4-{3-[4-(4-n-pentylbenzoyloxy)phenyl]-1,2,4-oxadiazol-5-yl}phenyl 4-hexylbenzoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 12h; Inert atmosphere; | 71% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 10h; |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; silver(I) acetate In 2,2,2-trifluoroethanol at 140℃; for 24h; | 71% |
-
-
950821-44-0
2-(2,4-difluorophenyl)-1-[4-(2-fluoro-4-amino-phenyl)-piperazin-1-yl]-3-(1H-1,2,4-triazol-1-yl)-propan-2-ol

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 68.5% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In chloroform at 20℃; for 48h; | 63% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
123843-59-4
4-cyano-3,5-difluoro-4'-hydroxybiphenyl

| Conditions | Yield |
|---|---|
| With 4-pyrrolidin-1-ylpyridine; dicyclohexyl-carbodiimide In dichloromethane Ambient temperature; | 61% |
-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 110℃; | 57.2% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
1415695-51-0
N-deacetylcolchicine trifluoroacetate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 3h; Inert atmosphere; | 53% |
-
-
837430-97-4
(1S,3R,4R,5R,6R,7R,8R,9S)-(9-hydroxymethylhexaspiro[2.0.0.0.0.0.2.1.1.1.1.1]pentadec-1-yl)methanol

-
-
26311-45-5
4-pentylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 12h; | 52% |
-
-
26311-45-5
4-pentylbenzoic acid

-
-
70568-47-7
4-hydroxybenzoic acid 4-cyanophenyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 24h; | 50% |
4-Pentylbenzoic acid Chemical Properties
Molecular Formula:C12H16O2
Molar mass:192.25424 g/mol
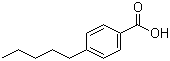
Structure of 4-Pentylbenzoic acid(26311-45-5):
Synonyms of 4-Pentylbenzoic acid(26311-45-5):4-n-Pentylbenzoic acid;p-n-Pentyl benzoic acid;Benzoic acid, 4-pentyl-;p-Pentylbenzoic acid;Para-Pentylbenzoic acid;4-Amylbenzoic acid;p-Amylbenzoic acid
Density:1.043 g/cm3
Flash Point:149.7 °C
Boiling Point:315.3 °C at 760 mmHg
Index of Refraction:1.526
Vapour Pressure:0.000187 mmHg at 25°C
Melting point:85-90°C
Water solubility:insoluble
4-Pentylbenzoic acid Uses
4-Pentylbenzoic acid(26311-45-5) can be used as intermediates of Liquid Crystals.
4-Pentylbenzoic acid Toxicity Data With Reference
| 1. | ivn-mus LD50:56 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#10073 . |
4-Pentylbenzoic acid Consensus Reports
4-Pentylbenzoic acid Safety Profile
Poison by intravenous route. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:Xi
Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
4-Pentylbenzoic acid Specification
Chemical Stability:Stable under normal pressures and temperatures.
Incompatibilities with Other Materials:Strong oxidizing agents.
Hazardous Decomposition Products: carbon monoxide, carbon dioxide.
Related Products
- 4-Pentylbenzoic acid
- 2631-37-0
- 2631-40-5
- 26314-06-7
- 263147-79-1
- 26315-07-1
- 26315-32-2
- 263155-11-9
- 263155-12-0
- 26315-61-7
- 263157-86-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View