-
Name
4-Piperidinopiperidine
- EINECS 225-522-8
- CAS No. 4897-50-1
- Article Data6
- CAS DataBase
- Density 0.969 g/cm3
- Solubility
- Melting Point 64-66 °C(lit.)
- Formula C10H20N2
- Boiling Point 251.4 °C at 760 mmHg
- Molecular Weight 168.282
- Flash Point 99.9 °C
- Transport Information
- Appearance light yellow to yellow-brown crystalline solid
- Safety 24/25-26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,4-Bipiperidyl;1,4'-bipiperidine;1-(3,4,5,6-tetrahydro-2H-pyridin-4-yl)-3,4,5,6-tetrahydro-2H-pyridine;4-(1-piperidinyl)piperidine;[1,4']Bipiperidinyl;4-(1-Piperidino)piperidine;1-(4-piperidyl)piperidine;
- PSA 15.27000
- LogP 1.49100
Synthetic route
-
-
110-89-4
piperidine

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In dichloromethane at 0 - 20℃; for 16h; | 80% |
-
-
383865-57-4
4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl-amine

-
A
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| A n/a B 35% |
-
-
97682-44-5, 130144-33-1
irinotecan

-
A
-
4897-50-1
4-piperidinopiperidin

-
B
-
86639-52-3, 110714-48-2, 130144-34-2
7-ethyl-10-hydroxycamptothecin

| Conditions | Yield |
|---|---|
| With carboxylesterase at 37℃; for 0.0833333h; pH=7.4; |
-
-
944411-93-2
[1,4']bipiperidinyl-1'-carboxylic acid (4-bromo-phenyl)amide

-
A
-
4897-50-1
4-piperidinopiperidin

-
C
-
1608129-84-5
C14H14N2O

-
D
-
106-40-1
4-bromo-aniline

| Conditions | Yield |
|---|---|
| Suzuki-Miyaura Coupling; |

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; sodium tris(acetoxy)borohydride / dichloromethane / 16 h / 0 - 20 °C 2: hydrogenchloride / methanol / 12 h / 40 °C View Scheme |
-
-
125541-12-0
tert-butyl 1,4’-bipiperidine-1’-carboxylate

-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 40℃; for 12h; | 2.04 g |
-
-
4897-50-1
4-piperidinopiperidin

-
-
2369-11-1
5-fluoro-2-nitroaniline

-
-
900506-32-3
2-nitro-5-(4-(piperidin-1-yl)piperidin-1-yl)benzenamine

| Conditions | Yield |
|---|---|
| With triethylamine In 1-methyl-pyrrolidin-2-one at 100℃; for 2.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one at 120℃; for 4h; | 75% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
5147-80-8
[Bis(methylthio)methylene]malononitrile

-
-
904677-87-8
[(methylthio)(4-piperidinopiperidino)methylene]malononitrile

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 0.25h; | 100% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
185321-62-4
2-tert-butyloxycarbonylamino-3-(3,4-dichlorophenyl)propionic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide | 100% |

| Conditions | Yield |
|---|---|
| 100% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
1361214-48-3
(R)-3-((2R,3S)-2-((E)-3-fluorostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoic acid

-
-
1361214-50-7
(R)-4-([1,4'-bipiperidin]-1'-yl)-2-((2R,3S)-2-((E)-3-fluorostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-N-((R)-1-phenylethyl)butanamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 18h; | 100% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
103816-19-9
4-piperidinopiperidine-1-carbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -10 - 20℃; for 4h; | 99% |
| Stage #1: 4-piperidinopiperidin; bis(trichloromethyl) carbonate In dichloromethane; acetonitrile at 20 - 70℃; Stage #2: With potassium carbonate In dichloromethane at 20℃; | 98.5% |
| Stage #1: 4-piperidinopiperidin; bis(trichloromethyl) carbonate In hexane; dichloromethane; acetonitrile at 20 - 25℃; Stage #2: With potassium carbonate In dichloromethane at 20℃; | 98.5% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
448-19-1
4-fluoro-2-methoxy-1-nitrobenzene

-
-
761440-29-3
1'-[3-(methyloxy)-4-nitrophenyl]-1,4'-bipiperidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 72h; | 99% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
169205-95-2
2-(methylthio)oxazolo[4,5-b]pyridine

-
-
1231933-81-5
C16H22N4O

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; | 99% |
| In N,N-dimethyl-formamide at 100℃; | 90% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
955084-10-3
5,5-difluoro-10-(p-tolyl)-5H-dipyrrolo[1,2-c:2',1'-f][1,3,2]diazaborinin-4-ium-5-uide

| Conditions | Yield |
|---|---|
| With silver(I) acetate In dimethyl sulfoxide at 80℃; for 12h; Schlenk technique; Inert atmosphere; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 60 - 65℃; for 3h; | 98.5% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-piperidinopiperidin; N-Boc pyroglutamic acid-Wang resin In tetrahydrofuran Addition; Stage #2: trifluoroacetic acid In water for 2.5h; Hydrolysis; | 98% |

-
-
82278-95-3
rac-(3-(3-bromophenyl)-2-tert-butoxycarbonylamino)propionic acid

-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide | 98% |
-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| Stage #1: 4-piperidinopiperidin; 2-{4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidin-1-yl}acetaldehyde With acetic acid In dichloromethane for 0.166667h; Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane for 17h; | 97.5% |
| Stage #1: 4-piperidinopiperidin; 2-{4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidin-1-yl}acetaldehyde With acetic acid In dichloromethane for 0.166667h; Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane for 17h; | 191.6 mg |
-
-
4897-50-1
4-piperidinopiperidin

-
-
1269606-87-2
2-[1,4']bipiperidinyl-1'-yl-1-(3-methoxy-phenyl)-ethanol

| Conditions | Yield |
|---|---|
| 97% |
-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 10h; | 97% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
49627-27-2
sulindac sulfide

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In acetonitrile at 20℃; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 2h; | 96% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
865626-93-3
(R)-2-(4-(8-Fluoro-2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carbonyloxy)-3-(7-methyl-2-((2-(trimethylsilyl)ethoxy)methyl)-2H-indazol-5-yl)propanoic acid

-
-
865626-94-4
(R)-3-(7-Methyl-2-((2-(trimethylsilyl)ethoxy)methyl)-2H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl 4-(8-fluoro-2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (R)-2-(4-(8-Fluoro-2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carbonyloxy)-3-(7-methyl-2-((2-(trimethylsilyl)ethoxy)methyl)-2H-indazol-5-yl)propanoic acid With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In DMF (N,N-dimethyl-formamide) at 20℃; for 0.0833333h; Stage #2: 4-piperidinopiperidin In DMF (N,N-dimethyl-formamide) at 20℃; for 2h; | 96% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
19313-88-3
3-chloro-N-(4-nitrophenyl)propanamide

-
-
1586764-66-0
3-([1,4'-bipiperidin]-1'-yl)-N-(4-nitrophenyl)propanamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; | 96% |
-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 10h; | 96% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 2h; | 96% |
| With potassium carbonate; potassium iodide In acetonitrile for 5h; | 92% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
89336-46-9
2-{2[(tert-butoxycarbonyl)amino]-1,3-thiazol-4-yl}acetic acid

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 20℃; | 96% |
-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; | 95.3% |
-
-
4897-50-1
4-piperidinopiperidin

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 60℃; for 1h; | 95.1% |
-
-
4897-50-1
4-piperidinopiperidin

-
-
583051-10-9
7-chloro-3,6-dicyano-2-ethoxy-4-phenyl-1,8-naphthyridine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 16h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide | 95% |
-
-
873090-18-7
5-(3-bromophenyl)-3H-1,3,4-oxadiazol-2-one

-
-
4897-50-1
4-piperidinopiperidin

-
-
1024613-87-3
1'-[5-(3-bromophenyl)-1,3,4-oxadiazol-2-yl]-1,4'-bipiperidinyl

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 18h; | 95% |
4-Piperidinopiperidine Specification
The 4-Piperidinopiperidine, with the CAS registry number 4897-50-1, is also known as 1,4'-Bipiperidin. It belongs to the product categories of Nitrogen Cyclic Compounds; Pharmaceutical Intermediates; Heterocycles; Piperidine; Heterocyclic Compounds; Piperidine Series; Piperidines. Its EINECS registry number is 225-522-8. This chemical's molecular formula is C10H20N2 and molecular weight is 168.2792. Its IUPAC name is called 1-piperidin-4-ylpiperidine. What's more, the product should be sealed and stored in cool and dry place.
Physical properties of 4-Piperidinopiperidine: (1)ACD/LogP: 1.53; (2)ACD/LogD (pH 5.5): -2.57; (3)ACD/LogD (pH 7.4): -2.18; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.5; (12)Molar Refractivity: 51.07 cm3; (13)Molar Volume: 173.6 cm3; (14)Surface Tension: 35.5 dyne/cm; (15)Density: 0.969 g/cm3; (16)Flash Point: 99.9 °C; (17)Enthalpy of Vaporization: 48.88 kJ/mol; (18)Boiling Point: 251.4 °C at 760 mmHg; (19)Vapour Pressure: 0.0205 mmHg at 25°C.
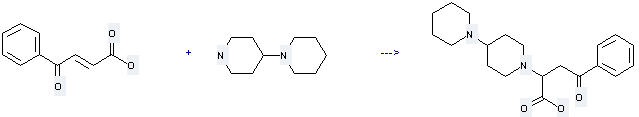
Uses of 4-Piperidinopiperidine: it can be used to produce 4-Oxo-4-phenyl-2-(4-piperidinopiperidino)-butansaeure with 4-oxo-4-phenyl-but-2-enoic acid. This reaction will need solvents toluene and ethanol. The yield is about 68%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1CCN(CC1)C2CCNCC2
(2)InChI: InChI=1S/C10H20N2/c1-2-8-12(9-3-1)10-4-6-11-7-5-10/h10-11H,1-9H2
(3)InChIKey: QDVBKXJMLILLLB-UHFFFAOYSA-N
Related Products
- 4-Piperidinopiperidine
- 4897-68-1
- 4897-84-1
- 489-84-9
- 4899-82-5
- 489-98-5
- 490021-97-1
- 490025-11-1
- 490028-21-2
- 490033-00-6
- 4900-33-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View