-
Name
5-Bromo-2,4-dichloropyrimidine
- EINECS -0
- CAS No. 36082-50-5
- Article Data22
- CAS DataBase
- Density 1.965 g/cm3
- Solubility
- Melting Point 29-30 °C(lit.)
- Formula C4HBrCl2N2
- Boiling Point 287.476 °C at 760 mmHg
- Molecular Weight 227.875
- Flash Point 127.661 °C
- Transport Information UN 3263 8/PG 2
- Appearance light yellow solid or liquid
- Safety 26-27-36/37/39-45
- Risk Codes 23/24/25-34
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 2,4-dichloro-5-bromo pyrimidine;5-bromo-2,4-dichloro-pyrimidine;2,4-Dichloro-5-bromo pyrimidine;Pyrimidine, 5-bromo-2,4-dichloro-;
- PSA 25.78000
- LogP 2.54590
Synthetic route
-
-
51-20-7
5-bromouracil

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In 1,1,2-trichloroethane Reagent/catalyst; Solvent; Reflux; | 99.5% |
| With N,N-dimethyl-aniline; trichlorophosphate at 120 - 130℃; for 1.33333h; | 96.6% |
| With phosgene; Tributylphosphine oxide at -5 - 125℃; for 1.83333h; Inert atmosphere; | 89.7% |
-
-
51-20-7
2,4-dihydroxy-5-bromopyrimidine

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With pyridine; trichlorophosphate at 160℃; for 2h; Autoclave; neat (no solvent); | 91% |
| With dmap; thionyl chloride; bis(trichloromethyl) carbonate for 12.5h; Reflux; Green chemistry; | 90% |
| With phosphorus pentachloride; trichlorophosphate |
-
-
51-20-7
5-bromouracil

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
10025-87-3, 12599-09-6, 63736-95-8
trichlorophosphate

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
113138-12-8
5-bromo-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; trichlorophosphate at 90℃; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
205672-25-9
4-amino-5-bromo-2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; | 100% |
| With ammonium hydroxide In tetrahydrofuran at 20℃; for 12h; | 100% |
| With ammonium hydroxide In tetrahydrofuran; water at 20℃; for 1.5h; | 100% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
844843-37-4
5-bromo-2-chloro-pyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 6h; Cooling; | 86% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With sodium hydroxide In tetrahydrofuran at 20℃; for 3h; Stage #2: With hydrogenchloride In tetrahydrofuran | 64% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With sodium hydroxide In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; | 64% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
1197953-47-1
(2-aminophenyl)dimethyl phosphorus oxide

-
-
1407520-11-9
(2-((5-bromo-2-chloropyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In N,N-dimethyl-formamide at 65℃; for 8h; | 72% |
| With potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate In N,N-dimethyl-formamide at 65℃; for 7h; | 66% |
| With tetrabutylammonium hydrogensulfate; potassium carbonate In N,N-dimethyl-formamide at 65℃; for 7h; | 66% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 12h; | 2.96 g |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
75178-96-0
N-Boc-1,3-diaminopropane

-
-
1046784-89-7
(3-((5-bromo-2-chloropyrimidin-4-yl)amino)propyl)carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 3.5h; | 100% |
| With triethylamine In acetonitrile at 20℃; for 0.5h; | 41% |
| With triethylamine In acetonitrile at 0 - 20℃; | |
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol regioselective reaction; | |
| With potassium carbonate In isopropyl alcohol at 80℃; for 24h; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
68621-88-5
(3-aminophenyl)carbamic acid tert-butyl ester

-
-
1202760-30-2
tert-butyl (3-((5-bromo-2-chloropyrimidin-4-yl)amino)phenyl)carbamate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 21℃; for 16h; Inert atmosphere; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 98% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
74-89-5
methylamine

-
-
205672-24-8
5-bromo-2-chloro-N-methyl-pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; for 3.66667h; | 100% |
| In methanol; water Cooling with ice; | 88% |
| In methanol; ethanol at 0 - 20℃; for 3h; | 88% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
87120-72-7
1-(tert-butoxycarbonyl)-4-aminopiperidine

-
-
954221-10-4
tert-butyl 4-((5-bromo-2-chloropyrimidin-4-yl)amino)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 0.5h; | 82% |
| With trimethylamine In acetonitrile at 20℃; for 2h; | 82% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 20℃; | 67% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
100-51-6
benzyl alcohol

-
-
41244-53-5
2,4-bis(benzyloxy)-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 20 - 25℃; for 12h; | 93% |
| With sodium hydride In toluene at 20℃; for 18h; | 91% |
| With sodium hydride 1.) toluene, 50 deg C, 2.) toluene, 25 deg C, overnight; Yield given. Multistep reaction; | |
| With sodium hydride |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
13325-10-5
4-Aminobutanol

-
-
477593-33-2
4-((5-bromo-2-chloropyrimidin-4-yl)amino)butan-1-ol

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 0 - 20℃; for 16h; | 98% |
| With triethylamine In acetonitrile at 0 - 20℃; for 16h; | 98% |
| With triethylamine In butan-1-ol at 125℃; for 1h; Inert atmosphere; Microwave irradiation; | 77% |
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 80℃; for 0.333333h; Microwave irradiation; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
147081-44-5
(3S)-3-amino-1-(tert-butoxycarbonyl)-pyrrolidine

-
-
1146159-74-1
(S)-tert-butyl 3-(5-bromo-2-chloropyrimidin-4-ylamino)pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane at 10 - 20℃; for 20h; | 67% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In acetonitrile at 0 - 20℃; | |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 8h; | |
| With potassium carbonate In acetonitrile at 20℃; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
1021268-16-5
1-(5-bromo-2-chloropyrimidine-4-yl)hydrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol for 12h; Reflux; Inert atmosphere; | 100% |
| With hydrazine hydrate In methanol at 20℃; for 1h; Cooling with ice; | 87% |
| With hydrazine hydrate In ethanol at 5 - 20℃; for 1h; | 75% |
| With hydrazine hydrate; triethylamine In ethanol at 20℃; for 1h; Cooling with ice; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
108-91-8
cyclohexylamine

-
-
864655-05-0
5-bromo-2-chloro-N-cyclohexylpyrimidin-4-amine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; for 12h; | 95% |
| With diisopropylamine In 1,4-dioxane at 20℃; for 6h; | 63% |
| With sodium hydrogencarbonate In methanol at 20℃; for 6h; | 54% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
24347-58-8
(R,R)-2,3-butandiol

-
-
851008-29-2
(2R,3R)-3-[(5-bromo-2-chloropyrimidin-4-yl)oxy]butan-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: (R,R)-2,3-butandiol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.166667h; Stage #2: 2,4-dichloro-5-bromopyrimidine In tetrahydrofuran at 20℃; for 12h; | 81% |
| Stage #1: (R,R)-2,3-butandiol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.166667h; Stage #2: 2,4-dichloro-5-bromopyrimidine In tetrahydrofuran at 0 - 20℃; for 12h; | 81% |
| Stage #1: (R,R)-2,3-butandiol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.5h; Stage #2: 2,4-dichloro-5-bromopyrimidine In tetrahydrofuran at 0 - 20℃; for 12h; | 57% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
936920-11-5
N-{3-[(3-cyano-propyl)-methanesulfonyl-amino]-phenyl}-acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichloro-5-bromopyrimidine; N-{3-[(3-cyano-propyl)-methanesulfonyl-amino]-phenyl}-acetamide With hydrogenchloride; hydrogen; platinum(IV) oxide In ethanol; water at 20℃; under 760.051 Torr; for 5h; Stage #2: 2,4-dichloro-5-bromopyrimidine With triethylamine In acetonitrile at 20℃; for 16h; | 43% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
22483-09-6
2,2-dimethoxyethylamine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 20℃; for 24h; | 83% |
| With triethylamine In ethanol at 0 - 20℃; for 12h; | 77% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine; 2,2-dimethoxyethylamine In ethanol at 0℃; for 0.166667h; Inert atmosphere; Stage #2: With triethylamine In ethanol Inert atmosphere; | 64% |
| With triethylamine In ethanol at 0 - 20℃; for 16.25h; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
14316-06-4
D-alanine methyl ester hydrochloride

-
-
851008-31-6
methyl N-(5-bromo-2-chloropyrimidin-4-yl)-D-alaninate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; for 48h; | 86% |
| With triethylamine In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 48h; | 86% |
| With triethylamine In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; for 48h; | 86% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
554-84-7
meta-nitrophenol

| Conditions | Yield |
|---|---|
| Stage #1: meta-nitrophenol With sodium hydride In dimethyl sulfoxide; N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 2,4-dichloro-5-bromopyrimidine In dimethyl sulfoxide; N,N-dimethyl-formamide at 20℃; | 93% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With N-ethyl-N,N-diisopropylamine In ethanol at 0 - 20℃; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
371-40-4
4-fluoroaniline

-
-
280582-07-2
5-bromo-2-chloro-4-[(4-fluorophenyl)amino]-pyrimidine

| Conditions | Yield |
|---|---|
| With sodium acetate In tetrahydrofuran; water at 20℃; for 18h; | 90% |
| With potassium carbonate In water; isopropyl alcohol at 20℃; for 18h; | 90% |
| With sodium carbonate In ethanol at 20℃; for 16h; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
124-41-4
sodium methylate

-
-
57054-92-9
5-bromo-2-chloro-4-methoxypyrimidine

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 4h; | 93% |
| In methanol at 20℃; for 4h; Cooling with ice; | 93% |
| In methanol at 20℃; for 12h; | 90% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
144222-22-0
tert-butyl 4-(aminomethyl)piperidine-1-carboxylate

-
-
1289114-86-8
4-[(5-bromo-2-chloro-pyrimidin-4-ylamino)methyl]-piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With trimethylamine In acetonitrile at 20℃; for 2h; | 92% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 50℃; for 2h; Inert atmosphere; | 75% |
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 80℃; for 0.333333h; Microwave irradiation; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In butan-1-ol at 100℃; | 72% |
| With potassium carbonate In water; isopropyl alcohol at 20℃; | 51.4% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine; 4-methoxy-aniline In isopropyl alcohol at 20℃; Stage #2: With potassium carbonate In water at 20℃; | 51.4% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
934-22-5
5-aminobenzimidazole

-
-
852357-08-5
(1H-Benzoimidazol-5-yl)-(5-bromo-2-chloro-pyrimidin-4-yl)-amine

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol at 20℃; for 24h; | 93% |
| With sodium carbonate In ethanol at 20℃; for 16h; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
5813-64-9
2,2-dimethylpropylamine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 2h; | 70% |
| With sodium hydrogencarbonate In methanol at 20℃; for 6h; | |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 2h; |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
5188-07-8
sodium thiomethoxide

-
-
59549-51-8
5-bromo-2-chloro-4-(methylthio)pyrimidine

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 82.8% |
| In acetonitrile at 20℃; for 24h; | 70% |
| In acetonitrile at 20℃; for 24h; | 70% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
871254-61-4
2,4-dichloro-5-pyrimidinecarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichloro-5-bromopyrimidine With TurboGrignard In tetrahydrofuran at -78℃; for 2h; Bouveault Aldehyde Synthesis; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78 - -42℃; for 12h; | 38% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With isopropylmagnesium chloride; lithium chloride In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78 - -35℃; for 4h; | 31% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With hydrogenchloride In tetrahydrofuran at -78 - 35℃; for 0.5h; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78℃; for 2h; | 28% |
-
-
4394-85-8
4-morpholinecarboxaldehyde

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
871254-61-4
2,4-dichloro-5-pyrimidinecarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichloro-5-bromopyrimidine With TurboGrignard In tetrahydrofuran at -78 - -42℃; for 1.25h; Stage #2: 4-morpholinecarboxaldehyde In tetrahydrofuran at -78 - -42℃; for 3.91667h; | 87% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With TurboGrignard In tetrahydrofuran at 78℃; for 1h; Stage #2: 4-morpholinecarboxaldehyde In tetrahydrofuran at -78 - -35℃; for 1.5h; Stage #3: With hydrogenchloride; water In tetrahydrofuran at -35℃; | 71% |
-
-
120-72-9
indole

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
1356962-87-2
3-(5-Bromo-2-chloropyrimidin-4-yl)-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichloro-5-bromopyrimidine With aluminum (III) chloride In 1,2-dichloro-ethane at 80℃; for 0.5h; Stage #2: indole In 1,2-dichloro-ethane at 80℃; for 18h; | 98% |
| Stage #1: indole With methylmagnesium bromide In tetrahydrofuran; diethyl ether at 0℃; for 0.75h; Inert atmosphere; Stage #2: 2,4-dichloro-5-bromopyrimidine In tetrahydrofuran; diethyl ether at 0 - 60℃; for 2.25h; Inert atmosphere; | 75% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
1386399-13-8
(3-bromo-phenyl)-(2,4-dichloro-pyrimidin-5-yl)-methanol

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichloro-5-bromopyrimidine With isopropylmagnesium chloride In tetrahydrofuran at -35℃; for 1h; Inert atmosphere; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -35℃; for 2h; Inert atmosphere; Stage #3: With water In tetrahydrofuran Inert atmosphere; | 98.8% |
| Stage #1: 2,4-dichloro-5-bromopyrimidine With isopropylmagnesium chloride In tetrahydrofuran at -35℃; for 1h; Inert atmosphere; Stage #2: m-bromobenzoic aldehyde at -35℃; for 2h; | 98.8% |
-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

-
-
1289198-78-2
tert-butyl 4-(5-bromo-2-chloropyrimidin-4-yl)piperazine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 30℃; for 2h; | 89% |
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 80℃; for 0.333333h; Microwave irradiation; |
5-Bromo-2,4-dichloropyrimidine Standards and Recommendations
5-Bromo-2,4-dichloropyrimidine Specification
The 5-Bromo-2,4-dichloropyrimidine, with the CAS registry number 36082-50-5, is also called 2,4-Dichloro-5-bromo pyrimidine. And the molecular formula of this chemical is C4HBrCl2N2. It is a kind of colourless oil, and belongs to the following product categories: Pyrimidine Series; Pyridines, Pyrimidines, Purines and Pteredines; Pharmacetical; Halides; Halogenated; Organohalides; Nucleotides and Nucleosides; Aromatics Compounds; Bases & Related Reagents; Aromatics; Building Blocks; Halogenated Heterocycles; Heterocyclic Building Blocks.
The physical properties of 5-Bromo-2,4-dichloropyrimidine are as following: (1)ACD/LogP: 1.89; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.891; (4)ACD/LogD (pH 7.4): 1.891; (5)ACD/BCF (pH 5.5): 16.103; (6)ACD/BCF (pH 7.4): 16.103; (7)ACD/KOC (pH 5.5): 254.408; (8)ACD/KOC (pH 7.4): 254.408; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 25.78 Å2; (13)Index of Refraction: 1.605; (14)Molar Refractivity: 39.916 cm3; (15)Molar Volume: 115.967 cm3; (16)Polarizability: 15.824×10-24cm3; (17)Surface Tension: 56.402 dyne/cm; (18)Density: 1.965 g/cm3; (19)Flash Point: 127.661 °C; (20)Enthalpy of Vaporization: 50.553 kJ/mol; (21)Boiling Point: 287.476 °C at 760 mmHg; (22)Vapour Pressure: 0.004 mmHg at 25°C.
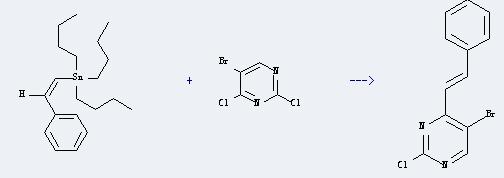
Uses of 5-Bromo-2,4-dichloropyrimidine: It can react with tributyl-styryl-stannane to produce 5-bromo-2-chloro-4-(b-styryl)pyrimidine. This reaction will need reagent dichlorobis(triphenylphosphine)palladium(II), and the solvent dimethylformamide. The reaction time is 6 hours with temperature of 70°C, and the yield is about 73%.
You should be cautious while dealing with this chemical. It is toxic by inhalation, in contact with skin and if swallowed, and it may also cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Take off immediately all contaminated clothing; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: c1c(c(nc(n1)Cl)Cl)Br
(2)InChI: InChI=1/C4HBrCl2N2/c5-2-1-8-4(7)9-3(2)6/h1H
(3)InChIKey: SIKXIUWKPGWBBF-UHFFFAOYAQ
Related Products
- 5-Bromo-2-(1-piperazinyl)pyrimidine
- 5-Bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)pyrimidine
- 5-Bromo-2-(2,5-dimethylpyrrol-1-yl)pyridine
- 5-Bromo-2-(2-methyl-2H-tetrazol-5-yl)-pyridine
- 5-Bromo-2(3H)-benzothiazolone
- 5-Bromo-2-(cyclobutyl)pyrimidine
- 5-Bromo-2-(cyclohexyl)pyrimidine
- 5-Bromo-2-(cyclopentyl)pyrimidine
- 5-Bromo-2-(difluoromethoxy)pyridine
- 5-Bromo-2-(hydroxymethyl)phenol
- 36083-56-4
- 36083-80-4
- 36085-64-0
- 36085-73-1
- 3608-58-0
- 36087-09-9
- 36087-94-2
- 3608-86-4
- 36092-42-9
- 360-92-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View