-
Name
2-Amino-3,6,8-naphthalenetrisulfonic acid
- EINECS 204-229-9
- CAS No. 118-03-6
- Article Data13
- CAS DataBase
- Density 1.974 g/cm3
- Solubility 1000g/L at 25℃
- Melting Point 140-143°C(lit.)
- Formula C10H9NO9S3
- Boiling Point 702.53℃[at 101 325 Pa]
- Molecular Weight 383.381
- Flash Point >230°F
- Transport Information
- Appearance
- Safety
- Risk Codes R36/37/38; R22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 1,3,6-Naphthalenetrisulfonic acid, 7-amino-;7-Aminonaphthalene-1,3,6-trisulphonic acid;2-Amino-3,6,8-naphthalenetrisulfonic acid;2-Naphthylamine-3,6,8-trisulfonic acid;Kyselina 2-naftylamin-3,6,8-trisulfonova;Kyselina kochova;BRN 2681042;NSC 7561;
- PSA 214.27000
- LogP 3.98570
Synthetic route

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 50℃; for 6.89h; electrochemical reduction; | 98.1% |
-
-
6259-66-1
7-hydroxy-naphthalene-1,3,6-trisulfonic acid

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| With ammonia |

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 120 - 130℃; |
-
-
38267-31-1
8-nitronaphthalene-1,3,6-trisulphonic acid

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| With ammonia unter Druck; |

| Conditions | Yield |
|---|---|
| at 120 - 130℃; |
-
-
38267-31-1
8-nitronaphthalene-1,3,6-trisulphonic acid

-
-
7664-41-7
ammonia

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| at 100℃; unter Druck; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid / 40 - 60 °C 2: potassium chloride; ammonia; ammonium hydrogen sulfite / water / 130 °C 3: sulfuric acid / 130 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 130℃; Temperature; Inert atmosphere; | 195.3 g |
-
-
108-77-0
1,3,5-trichloro-2,4,6-triazine

-
-
25711-72-2
1-amino-3-ureidobenzene

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With hydrogenchloride; sodium carbonate; sodium nitrite In water for 1h; Stage #2: 1-amino-3-ureidobenzene With sodium carbonate In water at 0 - 5℃; for 2h; pH=6 - 6.5; Stage #3: 1,3,5-trichloro-2,4,6-triazine In water at 0 - 35℃; for 3h; pH=6 - 6.5; | 82% |


| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With lithium hydroxide In water pH=7 - 8; Stage #2: With hydrogenchloride; sodium nitrite In water at 0 - 10℃; for 1h; Stage #3: Cresidine With pyridine In water; acetone at 0 - 25℃; | 66% |
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With hydrogenchloride; sodium nitrite In water at 5 - 10℃; for 1h; Stage #2: Cresidine With hydrogenchloride; sodium carbonate In water at 10 - 30℃; pH=3; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
90-40-4
2-amino-8-hydroxy-naphthalene-3,6-disulphonic acid

| Conditions | Yield |
|---|---|
| With alkali at 220 - 260℃; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| durch folgeweise Diazotierung, Behandlung mit xanthogensaurem Kalium, Verseifung und Oxydation mittels KMnO4; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| Diazotization; |
-
-
38661-32-4
4-(2-Iodo-ethyl)-naphthalen-1-ol

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
41017-52-1
7-[1-Hydroxy-4-(2-iodo-ethyl)-naphthalen-2-ylazo]-naphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| (i) NaNO2, aq. HCl, (ii) /BRN= 2364430/, aq. NaOH; Multistep reaction; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
90-40-4
2-amino-8-hydroxy-naphthalene-3,6-disulphonic acid

| Conditions | Yield |
|---|---|
| at 220 - 260℃; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
34043-07-7
8-hydroxy-3,6-disulfo-naphthalene-2-diazonium-betaine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: alkali / 220 - 260 °C 2: Diazotization View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
1426257-63-7
C10H7N2O9S3(1+)*Cl(1-)
-
-
6375-47-9
3-amino-4-methoxyacetanilide

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
82-75-7
8-anilino-1-naphthalenesulfonate

-
-
939374-04-6
C29H24N6O14S4

| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With hydrogenchloride; sodium nitrite In water at 10 - 25℃; Stage #2: 3-amino-4-methoxyacetanilide With sodium carbonate In water at 15 - 25℃; pH=5.5; Stage #3: 8-anilino-1-naphthalenesulfonate With hydrogenchloride; sodium hydroxide; sodium nitrite more than 3 stages; |

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
102-28-3
m-acetamide aniline

-
-
93679-74-4
2-(4-amino-2-acetylaminophenylazo)-3,6,8-naphthalenetrisulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid Stage #2: m-acetamide aniline |
-
-
25711-72-2
1-amino-3-ureidobenzene

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
28566-82-7
C17H15N5O10S3

| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With sodium carbonate In water at 0℃; Stage #2: With hydrogenchloride; sodium nitrite In water at 5 - 10℃; for 0.5h; Stage #3: 1-amino-3-ureidobenzene With sodium carbonate In water for 0.5h; pH=5 - 6; | |
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With sodium carbonate In water at 0℃; Stage #2: With hydrogenchloride; sodium nitrite In water at 5 - 10℃; for 0.5h; Stage #3: 1-amino-3-ureidobenzene With sodium carbonate In water for 0.5h; pH=5 - 6; | |
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With sodium nitrite In water at 0 - 2℃; for 0.166667h; Stage #2: In water at 5 - 8℃; for 1.16667h; Acidic aqueous solution; Stage #3: 1-amino-3-ureidobenzene With sodium carbonate In water at 8 - 10℃; for 1.33333 - 1.5h; pH=4.0; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
101309-09-5
C26H25N5O11S3

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: lithium hydroxide / water / pH 7 - 8 1.2: 1 h / 0 - 10 °C 1.3: 0 - 25 °C 2.1: hydrogenchloride; sodium nitrite / water / 1.5 h / 0 - 5 °C 2.2: 0 - 25 °C / pH 6 View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: lithium hydroxide / water / pH 7 - 8 1.2: 1 h / 0 - 10 °C 1.3: 0 - 25 °C 2.1: hydrogenchloride; sodium nitrite / water / 1.5 h / 0 - 5 °C 2.2: 0 - 25 °C / pH 6 3.1: hydrogenchloride; sodium nitrite / 1.5 h / 0 - 5 °C 3.2: 2.25 h / 20 - 35 °C 3.3: 70 °C / pH 4.5 View Scheme |
-
-
6375-47-9
3-amino-4-methoxyacetanilide

-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
108094-73-1
C19H18N4O11S3

| Conditions | Yield |
|---|---|
| Stage #1: 7-aminonaphthalene-1,3,6-trisulfonic acid With hydrogenchloride; sodium nitrite In water at 8 - 15℃; for 1h; Cooling with ice; Stage #2: 3-amino-4-methoxyacetanilide With sodium hydroxide In water at 20℃; for 2h; pH=5 - 5.5; |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
939374-04-6
C29H24N6O14S4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / 8 - 15 °C / Cooling with ice 1.2: 2 h / 20 °C / pH 5 - 5.5 2.1: hydrogenchloride; sodium nitrite / water / 1 h / 5 - 10 °C 2.2: 2 h / 10 - 15 °C / pH 6 - 6.5 View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
1025062-43-4
C32H23Cl2N9O14S4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / 8 - 15 °C / Cooling with ice 1.2: 2 h / 20 °C / pH 5 - 5.5 2.1: hydrogenchloride; sodium nitrite / water / 1 h / 5 - 10 °C 2.2: 2 h / 10 - 15 °C / pH 6 - 6.5 3.1: sodium carbonate / water / 1 h / 0 - 15 °C / pH 6 - 7 View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / 8 - 15 °C / Cooling with ice 1.2: 2 h / 20 °C / pH 5 - 5.5 2.1: hydrogenchloride; sodium nitrite / water / 1 h / 5 - 10 °C 2.2: 2 h / 10 - 15 °C / pH 6 - 6.5 3.1: sodium carbonate / water / 1 h / 0 - 15 °C / pH 6 - 7 4.1: ammonia / water / 1 h / 40 - 45 °C View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

-
-
1403244-25-6
C38H30N11O16S4(1+)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / 8 - 15 °C / Cooling with ice 1.2: 2 h / 20 °C / pH 5 - 5.5 2.1: hydrogenchloride; sodium nitrite / water / 1 h / 5 - 10 °C 2.2: 2 h / 10 - 15 °C / pH 6 - 6.5 3.1: sodium carbonate / water / 1 h / 0 - 15 °C / pH 6 - 7 4.1: ammonia / water / 1 h / 40 - 45 °C 5.1: water / 5 h / 80 - 90 °C View Scheme |
-
-
118-03-6
7-aminonaphthalene-1,3,6-trisulfonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / 8 - 15 °C / Cooling with ice 1.2: 2 h / 20 °C / pH 5 - 5.5 2.1: hydrogenchloride; sodium nitrite / water / 1 h / 5 - 10 °C 2.2: 2 h / 10 - 15 °C / pH 6 - 6.5 3.1: sodium carbonate / water / 1 h / 0 - 15 °C / pH 6 - 7 4.1: water / 1 h / 40 - 45 °C View Scheme |
7-Amino-1,3,6-naphthalenetrisulfonic acid Consensus Reports
7-Amino-1,3,6-naphthalenetrisulfonic acid Specification
The 7-Amino-1,3,6-naphthalenetrisulfonic acid with CAS registry number of 118-03-6 is also known as 1,3,6-Naphthalenetrisulfonic acid, 7-amino-. The IUPAC name is 7-Aminonaphthalene-1,3,6-trisulfonic acid. It belongs to product categories of Intermediates of Dyes and Pigments; Organic Acids. Its EINECS registry number is 204-229-9. In addition, the formula is C10H9NO9S3 and the molecular weight is 383.37. This chemical should be stored in ventilated, cool and dry room. What's more, it is used for manufacture of dyes and acid-2R.
Physical properties about 7-Amino-1,3,6-naphthalenetrisulfonic acid are: (1)XLogP3-AA: -0.7; (2)H-Bond Donor: 4; (3)H-Bond Acceptor: 10; (4)Rotatable Bond Count: 3; (5)Exact Mass: 382.943943; (6)MonoIsotopic Mass: 382.943943; (7)Topological Polar Surface Area: 214; (8)Heavy Atom Count: 23; (9)Complexity: 756; (10)Covalently-Bonded Unit Count: 1.
Preparation of 7-Amino-1,3,6-naphthalenetrisulfonic acid: it is prepared by reaction of 1-nitronaphthalene-3,6,8-trisulfonic acid. The reaction needs reagent aq. H2SO4 at the temperature of 50 °C with other condition of electrochemical reduction for 6.89 hours. The yield is about 98.1%.
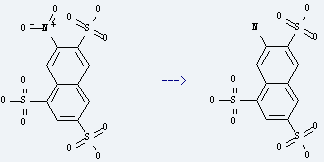
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=C2C=C(C=C(C2=CC(=C1S(=O)(=O)O)N)S(=O)(=O)O)S(=O)(=O)O
2. InChI: InChI=1S/C10H9NO9S3/c11-8-4-7-5(2-10(8)23(18,19)20)1-6(21(12,
13)14)3-9(7)22(15,16)17/h1-4H,11H2,(H,12,13,14)(H,15,16,17)(H,18,19,20)
3. InChIKey: GFPQSWFFPRQEHH-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 13gm/kg (13000mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1058, 1986. |
Related Products
- 7-Amino-1,2,3,4-tetrahydroisoquinoline
- 7-Amino-1,2,3,4-tetrahydroquinoline
- 7-Amino-1,3,6-naphthalenetrisulfonic acid
- 7-Amino-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one
- 7-Amino-1,3-naphthalenedisulfonic acid
- 7-Amino-1H-benzimidazole-5-carboxylic acid
- 7-Amino-1H-indole-2-carboxylic acid
- 7-Amino-1-methyl-1,2,3,4-tetrahydroquinoline
- 7-Amino-1-naphthalenesulfonic acid
- 1180-43-4
- 118-04-7
- 1180-60-5
- 118062-05-8
- 118068-30-7
- 118-07-0
- 1180-71-8
- 118072-93-8
- 118078-66-3
- 118081-34-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View