-
Name
ALLYLDIMETHYLCHLOROSILANE
- EINECS 223-706-2
- CAS No. 4028-23-3
- Article Data21
- CAS DataBase
- Density 0.879 g/cm3
- Solubility Soluble in most organic solvents; reacts with alcohols, amines, and water.
- Melting Point
- Formula C5H11ClSi
- Boiling Point 114.5 °C at 760 mmHg
- Molecular Weight 134.681
- Flash Point 5.6 °C
- Transport Information UN 2985 3/PG 2
- Appearance clear colorless to light yellow liquid
- Safety 16-26-36/37/39-45
- Risk Codes 11-34
-
Molecular Structure
-
Hazard Symbols
 F,
F,  C
C
- Synonyms Silane,allylchlorodimethyl- (6CI,7CI,8CI);Silane, chlorodimethyl-2-propenyl- (9CI);Allyldimethylchlorosilane;Allyldimethylsilylchloride;Chlorodimethyl(2-propenyl)silane;
- PSA 0.00000
- LogP 2.61630
Synthetic route
-
-
78847-25-3
allyl(2-chloropropyl)dimethylsilane

-
A
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| Heating; | A 95% B 5% |
-
-
75-78-5
dimethylsilicon dichloride

-
-
106-95-6
allyl bromide

-
A
-
4028-23-3
Allylchlorodimethylsilane

-
B
-
1113-12-8
diallyl(dimethyl)silane

| Conditions | Yield |
|---|---|
| Stage #1: allyl bromide With indium In 1,3-dimethylimidazolidine at 20℃; for 1h; Stage #2: dimethylsilicon dichloride In 1,3-dimethylimidazolidine at 70℃; for 3h; | A 90% B 8% |

-
-
762-72-1
allyl-trimethyl-silane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
4028-23-3
Allylchlorodimethylsilane

-
C
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With tungsten(VI) oxychloride In benzene at 50℃; for 60h; | A 87% B 7% C 4% |

-
-
2622-05-1
allylmagnesium bromide

-
-
75-78-5
dimethylsilicon dichloride

-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 25℃; for 16h; | 80% |
-
-
1113-12-8
diallyl(dimethyl)silane

-
A
-
4028-23-3
Allylchlorodimethylsilane

-
B
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With tungsten(VI) oxychloride In benzene at 50℃; for 60h; | A 25% B 74% |
| With tungsten(VI) oxychloride In benzene at 50℃; for 60h; | A 25% B 74% |
-
-
75-78-5
dimethylsilicon dichloride

-
-
1730-25-2
allylmagnesium bromide

-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| In diethyl ether for 2h; Heating; | 66% |
| 28% | |
| In diethyl ether |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloroprop-1-ene With iodine; magnesium In diethyl ether at 20℃; for 12h; Stage #2: dimethylsilicon dichloride In diethyl ether at 20℃; for 18h; Further stages.; | 44% |
-
-
60-29-7
diethyl ether

-
-
107-37-9
allyltrichlorosilane

-
-
75-16-1
methylmagnesium bromide

-
A
-
4028-23-3
Allylchlorodimethylsilane

-
B
-
1873-92-3
allyldichloromethylsilane


| Conditions | Yield |
|---|---|
| With diethyl ether |
-
-
10605-40-0
(3-chloropropyl)dimethylchlorosilane

-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| With piperidine |
-
-
57522-83-5
Dimethylcyclopropylchlorosilane

-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| at 480℃; |

| Conditions | Yield |
|---|---|
| With magnesium In diethyl ether | |
| Stage #1: allyl bromide With 1,3-dimethyl-2-imidazolidinone; indium at 20℃; for 2h; Inert atmosphere; Stage #2: dimethylsilicon dichloride at 70℃; for 3h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In benzene Mechanism; |

| Conditions | Yield |
|---|---|
| at 590℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCl / 20 °C 2: 95 percent / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With CH3MgBr In not given |

| Conditions | Yield |
|---|---|
| at 520℃; Product distribution / selectivity; Inert atmosphere; | 58 %Chromat. |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
107-05-1
3-chloroprop-1-ene

-
A
-
4028-23-3
Allylchlorodimethylsilane

-
B
-
64472-98-6
Chloro-dimethyl-((E)-propenyl)-silane

| Conditions | Yield |
|---|---|
| at 500℃; Product distribution / selectivity; Inert atmosphere; | A 79 %Chromat. B 20 %Chromat. |


| Conditions | Yield |
|---|---|
| In diethyl ether at -20 - 20℃; for 16h; |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
4358-87-6
(RS)-methyl mandelate

-
-
123464-07-3
allyl<<α-(methoxycarbonyl)benzyl>oxy>dimethylsilane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 2h; Ambient temperature; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
140134-75-4
1-<5'-O-(monomethoxytrityl)-2'-deoxy-2'-phenylseleno-β-D-xylofuranosyl>thymine

-
-
140134-83-4
1-<5'-O-(monomethoxytrityl)-(3'-O-allyldimethylsilyl)-2'-deoxy-2'-phenylseleno-β-D-xylofuranosyl>thymine

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Ambient temperature; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
140134-77-6
1-<5'-O-(monomethoxytrityl)-2'-deoxy-2'-phenylseleno-β-D-ribofuranosyl>thymine

-
-
140134-90-3
1-<5'-O-(monomethoxytrityl)-(3'-O-allyldimethylsilyl)-2'-deoxy-2'-phenylseleno-β-D-ribofuranosyl>thymine

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Ambient temperature; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
140134-76-5
1-<5'-O-(monomethoxytrityl)-3'-deoxy-3'-phenylseleno-β-D-xylofuranosyl>thymine

-
-
1009794-94-8
1-<5'-O-(monomethoxytrityl)-(2'-O-allyldimethylsilyl)-3'-deoxy-3'-phenylseleno-β-D-ribofuranosyl>thymine

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Ambient temperature; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
140134-74-3
1-<5'-O-(monomethoxytrityl)-3'-deoxy-3'-phenylseleno-β-D-arabinofuranosyl>thymine

-
-
140134-80-1
1-<5'-O-(monomethoxytrityl)-(2'-O-allyldimethylsilyl)-3'-deoxy-3'-phenylseleno-β-D-arabinofuranosyl>thymine

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Ambient temperature; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
102632-99-5, 134679-60-0, 144178-31-4, 54525-86-9
1-phenyl-4-penten-1-ol

-
-
200192-52-5
Allyl-dimethyl-(1-phenyl-pent-4-enyloxy)-silane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
52922-10-8
(1SR,2SR)-2-methyl-1-phenylbut-3-en-1-ol

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
128775-56-4
phenyl 3,4,6-tri-O-benzyl-1-seleno-β-D-glucopyranoside

-
-
288585-01-3
phenyl 2-O-(allyl(dimethyl)silyl)-3,4,6-tri-O-benzyl-1-seleno-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In toluene at 20℃; Condensation; | 100% |
| With dmap; triethylamine In toluene at 20℃; for 3h; | 98% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole; N-ethyl-N,N-diisopropylamine In dichloromethane at -78 - 20℃; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
58931-16-1
1-(benzyloxy)-4-penten-2-ol

-
-
193557-34-5
benzyl 2-(allyldimethylsilyloxy)-4-penten-1-yl ether

| Conditions | Yield |
|---|---|
| With 1H-imidazole; N-ethyl-N,N-diisopropylamine In dichloromethane at -78 - 20℃; for 4h; | 100% |
-
-
763-32-6
2-methyl-1-buten-4-ol

-
-
4028-23-3
Allylchlorodimethylsilane

-
-
1012314-18-9
allyl(dimethyl)[(3-methylbut-3-en-1-yl)oxy]silane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 99% |
-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: ((2-bromo-4-methoxyphenyl)ethynyl)trimethylsilane With n-butyllithium In tetrahydrofuran at -78℃; for 1h; Stage #2: Allylchlorodimethylsilane In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: With potassium hydroxide In tetrahydrofuran; methanol at 20℃; for 0.166667h; | 100% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
4393-06-0
1-Phenyl-2-propen-1-ol

-
-
200192-50-3
allyldimethyl(1-phenylallyloxy)silane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 99% |
| With 1H-imidazole; triethylamine In dichloromethane at 0 - 20℃; for 1h; | 93% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
80735-94-0
1-Phenyl-3-buten-1-ol

-
-
200192-51-4
Allyl-dimethyl-(1-phenyl-but-3-enyloxy)-silane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 99% |
| With 1H-imidazole; triethylamine In dichloromethane at -10 - 20℃; for 1h; | 73% |
| With triethylamine In dichloromethane Yield given; |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
58931-16-1, 88981-35-5, 110339-28-1
(R)-1-benzyloxypent-4-en-2-ol

-
-
337983-96-7
Allyl-((R)-1-benzyloxymethyl-but-3-enyloxy)-dimethyl-silane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
67-63-0
isopropyl alcohol

-
-
98582-95-7
allyl(isopropoxy)dimethylsilane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromonaphthalene With n-butyllithium In tetrahydrofuran at -78℃; Stage #2: Allylchlorodimethylsilane In tetrahydrofuran at -78℃; for 5h; Further stages.; | 99% |
-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran for 12h; Ambient temperature; | 98% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
195875-43-5
(2R,3S,5aR,6aS,9R,10S,11aR,13aS)-2,10-Divinyl-tetradecahydro-1,6,11-trioxa-cyclohepta[b]heptalene-3,9-diol

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 25℃; for 12h; Etherification; | 98% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
183387-28-2
1,3,5,7,9,11,14-heptacyclopentyltricyclo[7.3.3.15,11]heptasiloxane-endo-3,7,14-triol

-
-
319910-52-6
(cyclopentyl)7Si7O11(OH)2(OSiMe2-allyl)

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 18h; | 98% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
183387-28-2
1,3,5,7,9,11,14-heptacyclopentyltricyclo[7.3.3.15,11]heptasiloxane-endo-3,7,14-triol

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 18h; | 98% |
-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole; N-ethyl-N,N-diisopropylamine In dichloromethane at -78 - 20℃; for 4h; | 98% |
-
-
246047-72-3
(1,3-dimesityl-4,5-dihydroimidazol-2-ylidene)(PCy3)Cl2Ru=CHPh

-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| In dichloromethane 22°C, 1 h; | 98% |

-
-
4028-23-3
Allylchlorodimethylsilane

-
-
94236-21-2
1-bromo-2-((methoxymethoxy)methyl)benzene

-
-
1380232-83-6
allyl[2-{(methoxymethoxy)methyl}phenyl]dimethylsilane

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-2-((methoxymethoxy)methyl)benzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: Allylchlorodimethylsilane at -78 - 20℃; | 98% |
-
-
4028-23-3
Allylchlorodimethylsilane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 97% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
95-56-7
2-hydroxybromobenzene

-
-
203064-84-0
Allyl-(2-bromo-phenoxy)-dimethyl-silane

| Conditions | Yield |
|---|---|
| With triethylamine at 30℃; for 0.333333h; | 97% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
60410-16-4
(1R,4S)-4-hydroxy-2-cyclopentene-1-yl acetate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25℃; for 3h; Substitution; | 97% |
-
-
4028-23-3
Allylchlorodimethylsilane

-
-
288584-99-6
phenyl 3,4-bis-O-tert-butyldimethylsilyl-6-O-trityl-1-seleno-β-D-glucose

-
-
288585-02-4
phenyl 2-O-(allyl(dimethyl)silyl)-3,4-bis-O-(tert-butyl(dimethyl)silyl)-6-O-(triphenylmethyl)-1-seleno-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With pyridine; 3 A molecular sieve at 20℃; for 1h; | 97% |
| With dmap; triethylamine In toluene Condensation; | 67% |
Allylchlorodimethylsilane Specification
The CAS register number of Allylchlorodimethylsilane is 4028-23-3. It also can be called as Silane, chlorodimethyl-2-propen-1-yl- and the IUPAC name about this chemical is chloro-dimethyl-prop-2-enylsilane. The molecular formula about this chemical is C5H11ClSi and molecular weight is 134.68. It belongs to the following product categories, such as Monomer; Pentafluorophenylsilylation, etc. (GC Derivatizing Reagents); Si-Cl Compounds; Silylation (GC Derivatizing Reagents); Analytical Chemistry; GC Derivatizing Reagents; Monochlorosilanes; Si (Classes of Silicon Compounds); Vinylsilanes, Allylsilanes and so on.
Physical properties about Allylchlorodimethylsilane are: (1)ACD/LogP: 3.00; (2)ACD/LogD (pH 5.5): 3; (3)ACD/LogD (pH 7.4): 3; (4)#Freely Rotating Bonds: 2; (5)Index of Refraction: 1.416; (6)Molar Refractivity: 38.5 cm3; (7)Molar Volume: 153.2 cm3; (8)Polarizability: 15.26x10-24cm3; (9)Surface Tension: 18.4 dyne/cm; (10)Enthalpy of Vaporization: 33.83 kJ/mol; (11)Boiling Point: 114.5 °C at 760 mmHg; (12)Vapour Pressure: 23.6 mmHg at 25°C.
Preparation: this chemical can be prepared by dichloro-dimethyl-silane and allylmagnesium bromide. This reaction will need solvent diethyl ether. The reaction time is 2 hour(s) at heating. The yield is about 66%.
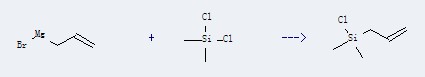
Uses of Allylchlorodimethylsilane: it can be used to produce allyldimethylsilyl benzhydryl ether with diphenylmethanol at heating. This reaction will need reagent Et3N and solvent diethyl ether with reaction time of 4 hours. The yield is about 83.2%.

When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and it can cause burns. When you are using it, wear suitable protective clothing, gloves and eye/face protection, you also need keep away from sources of ignition. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice and in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
You can still convert the following datas into molecular structure:
(1)SMILES: C=CC[Si](C)(C)Cl
(2)InChI: InChI=1/C5H11ClSi/c1-4-5-7(2,3)6/h4H,1,5H2,2-3H3
(3)InChIKey: KMVZWUQHMJAWSY-UHFFFAOYAR
(4)Std. InChI: InChI=1S/C5H11ClSi/c1-4-5-7(2,3)6/h4H,1,5H2,2-3H3
(5)Std. InChIKey: KMVZWUQHMJAWSY-UHFFFAOYSA-N
Related Products
- Allylchlorodimethylsilane
- 402824-96-8
- 40283-41-8
- 40283-46-3
- 402846-78-0
- 4028-63-1
- 40290-32-2
- 40291-39-2
- 4029-26-9
- 402927-97-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View